ISSN: 2320-0189
ISSN: 2320-0189
1Department of Chemistry, Abbottabad University of Science and Technology, Pakistan
2Department of Chemistry, COMSATS University Islamabad (CUI), Pakistan
3Department of Chemistry, University of Education, Pakistan
4Department of Chemistry, University of Swat (UoS), Pakistan
Received Date: 22/05/2020; Accepted Date: 24/07/2020; Published Date: 31/07/2020
Visit for more related articles at Research & Reviews: Journal of Botanical Sciences
Rising population has globally challenged the food security for all livings, which ultimately need the agricultural land expansion to meet the demands. For cultivations, along with other parameters, optimal availability of most of the nutrients is found at pH less than 6. And alkaline soils covering more than one fourth of the earth's surface constrain the wide range of available land for cultivations. Amendments in pH are usually brought out by the addition of either synthetic chemicals or natural biomass but, effectiveness of any amendment is directly resisted by soil’s buffering capacity. Buffering capacity retains the pH of the soil. This study is designed to lower the soil pH as well as to maintain it efficiently, over a long period of time by using various chemicals. Chemicals tried to reduce the soil pH in this study were: aluminum sulphate (Al2(SO4)3), hydrogen peroxide (H2O2), hydrochloric acid (HCl) and sulphuric acid (H2SO4). The chemicals were employed on three different textured soils in batch, both individually and in combinations. The buffering capacity of different soils was related with soil textures to compare the effects of chemical amendments on individual soils. The soil-water (1:1) paste was kept at room temperature for five months after the application of treatment. Most of the chemical treatments not only lowered the pH significantly but retained it as well for longer period of time, indicating suitability of cultivation at desired acidic pH, ranging from 3-6.
Soil pH, Chemical amendment, Soil texture, pH conditioning
Expanding global population and limited availability of arable land has made food security a major challenge for our planet [1]. To fulfill the food demands for growing population, there must be intensive agriculture production by increasing the percentage of dry land cultivation area. Significant efforts are being made to treat the limited available land not only to meet the required food targets but also to improve the rural income opportunities especially in developing countries [2]. Many regions of the world have potential to fulfill the requirements for cultivations except the basic pH range, which offers the constraint to exploit available land for agricultural purposes [3]. Soil pH, is a concentration of hydrogen ions and expressed on a negative log scale. One unit increase or decrease in pH value corresponds to ten-fold increase or decrease in hydrogen ion concentration [4]. pH of the soil lies usually between 3 and 8, with pH below 7.0 being acidic and above 7.0 being alkaline. All physical, chemical and biological properties of the soil are strongly influenced by soil pH [5,6]. And soil pH is a “master variable” that not only controls nutrient availability and microbial activities of the soil, but crop growth and plant development as well [7]. Soil texture, organic and inorganic nutrients and various chemical processes occurring in the soil are responsible to determine the fate of the pH of the soil [8]. pH variations are also influenced by parent materials of the soil and has a negative relation with mean precipitation and temperature [4]. Topographical aspect and slope are also the factors, affecting the soil pH. Literature reveals the potential of agricultural expansion in many areas of the world owing to the suitability of all cultivation parameters i.e. temperature, precipitation, etc. except alkaline nature of soil, due to which crop production is not feasible [3]. To bring the soil in desired pH range, required for specific plantation, amendments are done by the addition of either synthetic chemicals or natural biomass [9]. The effectiveness of any amendment is strongly dependent upon soil’s buffering capacity which in turn is dictated by several factors: e.g., mineralogy, soil texture, organic matter content and their complex combinations [10]. Soil buffering capacity ensures the stability in the pH of soil [11]. Strategies have been designing for lowering of soil pH by the help of natural as well as chemical modifications, but its long term stability is a difficult task to maintain for longer terms. Methods used for acidification include, either direct inorganic acidification i.e., H2SO4, HNO3, HCl or salts of trivalent metal ions e.g., aluminum (Al) and iron (Fe) and indirect biological assisted acidification of NH4 or S [12]. Addition of organic matter to the soil produces acid and humus on decomposition that enhances Cation exchange capacity and consequently increases the percentage of acid saturation [13]. Among other chemical modifications, elemental sulphur is generally used, though it’s cost effective and has long-term field scale applications but its oxidation to sulphuric acid by autotrophic bacteria is a very slow process [14]. Aluminum sulphate contains 14% water soluble sulphur and it can be used as a chemical amendment for lowering soil pH [15]. Iron sulfate may also be used to acidify the soils and reacts much faster than elemental sulphur. But the long term maintenance of soil pH with individual chemicals is debatable for two reasons, (a) pH change after the immediate treatment is abrupt and (b) high buffering capacity of the soils quickly counter balances the alteration in pH [16]. Therefore, there is a need to design the treatments by the combination of different chemicals in order to address these two issues. This study focuses to design a treatment for long-term modification of soil pH by the addition of various acidifying chemical agents and to investigate the relation between buffering capacities of soil and their texture. The aim is to design a suitable combination of chemicals which can lower pH as well as to maintain it efficiently over a long period of time. The soil pH was monitored for the period of five months. Three types of soil were analyzed viz. clay loam, silt loam and sandy clay loam in the current study.
Sample Collection
Three different type of composite soil samples were collected from a depth of 0-20 cm. Soil texture and taxonomy was determined using the procedure adopted by Jozefaciuk et al. as given in the Table 1 [17]. The soil samples were first air dried, ground well and were passed through 2 mm sieve.
| Characters | Soil A | Soil B | Soil C |
| Clay% | 26 | 35.6 | 16.6 |
| Silt% | 21.4 | 31.8 | 52 |
| Sand% | 52.6 | 32.6 | 31.4 |
| Texture | sandy clay loam | clay loam | silt loam |
Table 1. Texture of three different soil samples under investigation.
Soil Analysis
Different physico-chemical properties of soil i.e. pH, electrical conductivity, organic matter, lime, nitrates, phosphorus and potassium content were determined for three different textured soils. After textured evaluation, the soil samples were assigned as sandy clay loam, clay loam and silt loam as evident in Table 1. Soil pH was measured by making 1:1 soil-water suspension in 400 g of soil in a disposable plastic beaker. Saturated paste was kept overnight to allow the process of salt dissolution, equilibrium and aerobic dissolution [18].The Electrical Conductivity (EC) was measured in 1:5 soil-water suspension using EC meter [19]. Lime was determined using acid neutralization method [20]. Organic matter of soil and phosphorous was determined by the methods mentioned in the literature [21,22]. Available potassium was determined by flame photometry (Flame photometer, Spectrolab S20- 4, Wiltshire UK). Total nitrogen was determined by method designed by Breemmer (Breemmer 1982). The measured physicochemical properties are tabulated in Table 2.
| Property | Sandy clay loam | Clay loam | Silt loam |
|---|---|---|---|
| Electrical conductivity (dsm-1) | 0.32 | 0.61 | 0.42 |
| Organic matter (%) | 2.08 | 0.92 | 0.67 |
| Lime (%) | 3.4 | 4.9 | 6 |
| Nitrate (mg/kg soil) | 18.5 | 15.5 | 8 |
| P (mg/kg soil) | 4.9 | 3.5 | 4.7 |
| K (mg/kg soil) | 121 | 90 | 112 |
| pH | 7.48 | 6.44 | 6.74 |
Table 2. Physico-chemical properties of the soil samples under investigation.
Soil Treatment
After measuring the physico-chemical properties, the soils were subjected to various chemical treatments, applied both individually and in combinations, shown by Table 3. Amount of any chemical dose designed, depends on its prevailing as well as desired targeted pH and the buffering capacity of soil. All the chemicals used for the modification of soil pH were of analytical grade are as follows: aluminum sulphate (Al2(SO4)3, Sigma, 99.99%), hydrogen peroxide (H2O2, Fluka, ≥ 30%), hydrochloric acid (HCl, Sigma, 38%) and sulphuric acid (H2SO4, Sigma, 99.99%).
| Treatments | Chemicals Applied |
|---|---|
| Treatment 1 | Aluminum sulphate (Al2(SO4)3) 50 g in 400 g soil |
| Treatment 2 | Hydrogen peroxide (H2O2) 35 ml (33%) in 400 g soil-water (1:1) paste |
| Treatment 3 | Hydrochloric acid (HCl) 6 M(38 ml) g in 400 g soil-water (1:1) paste |
| Treatment 4 | Sulphuric acid (H2SO4) 37% (7 ml) g in 400 g soil-water (1:1) paste |
| Treatment 5 | Aluminum sulphate(Al2(SO4)3)+Hydrogen peroxide (H2O2) 50 g + 35 ml in 400 g soil-water (1:1) paste |
| Treatment 6 | Aluminum sulphate(Al2(SO4)3)+Hydrochloric acid (HCl) 50 g+38 ml(6.7N) in 400 g soil-water (1:1) paste |
| Treatment 7 | Aluminum sulphate(Al2(SO4)3)+Sulphuric acid (H2SO4) 50 g+37% (7 ml) in 400 g soil-water (1:1) paste |
| Treatment 8 | Hydrogen peroxide (H2O2)+Hydrochloric acid (HCl) 35 ml (33%)+6 M (38 ml) in 400 g soil-water (1:1) paste |
| Treatment 9 | Hydrogen peroxide (H2O2)+Sulphuric acid (H2SO4) 35 ml (33%)+37% (7 ml) in 400 g soil-water (1:1) paste |
| Treatment 10 | Aluminum sulphate(Al2(SO4)3)+Hydrogen peroxide (H2O2)+Sulphuric acid (H2SO4) 50 g+35 ml (33%)+6 M(7 ml) in 400 g soil-water (1:1) paste |
Table 3. Summary of the different chemical treatments applied to soil samples.
Treatments were applied in three replications after making the paste of 400 g soil with water in 1:1 in a plastic beaker at room temperature. One controlled sample of three soil textures was also preserved (without any treatment). Paste was allowed to stay overnight in order to allow the process of salt dissolution and equilibration and then pH readings were taken. At start, pH was measured on daily basis but after a month the measurements were done after three days and afterwards ten days. The changes in pH were measured over a period of one hundred and eighty days.
Effects of Individual Treatment on pH of Sandy Clay Loam, Clay Loam and Silt Loam
The initial pH of untreated soil of sandy clay loam, clay loam and silt loam was 7.50, 6.44 and 6.74, respectively. Effects of individual treatments on the pH of sandy clay loam are shown in Figure 1 (a). Al2(SO4)3 was applied alone and it lowered down the pH to 2.95 and successfully retained it as it went up high to 3.75 only after 180 days, showing that soil buffering capacity was overcome by the slow proton release from Al2(SO4)3.
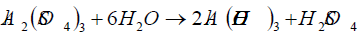 (1)
(1)
Direct application of an acid to the soil is the most effective to lower the pH of soil in no time [23]. H2SO4 and HCl addition reduced the pH to 3.87 and 0.38 from 7.48, which later on increased back up to 4.7 and 7.0 respectively due to buffering property of the soil as shown in Figure 1(a) [24]. H2O2 is a strong oxidizing agent, used for soil remediation by improving soil oxygenation [25].
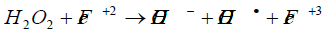 (2)
(2)
H2O2 upon natural decomposition dissociates into molecular oxygen needed for aerobic metabolism of microorganisms and roots. Oxygen availability is important as lack of soil oxygen directly affects root growth. Shoot and hence plant is growth is directly affected by soil oxygen [26]. The species like superoxide anion (O2-), hydroperoxyl radical (HO2º) and hydroperoxide anion (HO2-) are released in number of steps. The reactions are given below [27].
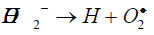 (3)
(3)
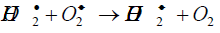 (4)
(4)
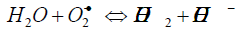 (5)
(5)
As shown in Figure 1 (a-c) H2O2 alone was not effective as it did not show pH lowering capability, due to possibility of degradation of organic matter of soil by the addition of H2O2 [27].
The pH of the clay loam was 6.44 before treatment. For clay loam all combinations were successful in lowering the pH significantly. Al2(SO4)3 alone reduced the pH from 6.44 to 2.72 and was successfully sustained for one eighty days. It shows that behavior of the clay loam is similar to the sandy clay loam but little variations were observed due to change in the texture of the soil as evident in Table 1. With the addition of acids i.e. H2SO4, reduction in pH to 1.73 was observed which fluctuated between 1.5 and 2.75 within 180 days. And with HCl, pH was reduced down to 0.67 and then reached up to 6.7 in 180 days by nullifying he affect of acid. Applying H2O2 into heavy clay soil, increases biomass and hence amount of crop production [28]. As discussed above, when H2O2 was used alone no change in pH was observed after twelve days but then increase in pH was shown and it reached up to pH 8.0, the results are shown in Figure 1 (b). This increase in pH was due to the destruction of organic matter, which contribute to resist the change in pH range [29]. The same results were obtained as for the study of sandy clay loam. High buffering capacity of this soil is attributed to high clay content of sandy clay loam as compared to clay and silt loam which is 35.61, 26 and 16.6%. Almost the same results with little variations were observed for sandy clay loam. In fact amount of any treatment is dependent on soil texture as clay and organic matter act as buffer by absorbing and releasing mineral ions therefore soils in high clay or organic matter soil respond little against any amendment.
For silt loam, Al2(SO4)3 reduced the pH from 6.74 to 3.65 and was successfully sustained for one eighty days as shown in Figure 1 (c). Addition of HCl and H2SO4 conditioned the pH to 6.6, 4.5 respectively. For H2O2, the same trend was observed as for sandy clay loam and sandy loam. It can be deduced from the Figure 1 (c) that the natural buffering capacity of soil is very small due to high sand content in soil and control of such type of soil is not feasible as are strongly affected by any type of amendments. As discussed previously, variations in results of three studied soil samples are due to the texture and component of soil [10].
Treatments Applied in Combination of Two Chemicals to Sandy Clay Loam, Clay Loam and Silt Loam
Treatments were applied in combination of two chemicals as well, the results are shown in Figure 2 (a-c). And when Al2(SO4)3 was used in the combination with acids, pH values were lowered to 2.0-2.5. In combination of two chemicals i.e. Al2(SO4)3 and H2O2 did not show any pH lowering capability but in fact, it reduced the pH lowering capability of Al2(SO4)3. The pH for Al2(SO4)3was found in the range of 3-3.5 while in the combination with H2O2, pH was raised up to 6.0-6.5. It seems that H2O2 mineralized the organic matter and due to lack of organic acid, rise in pH was observed. For clay loam, when Al2(SO4)3 was used in the combination with acids i.e. H2SO4 and HCl, a drastic decrease in pH (1.0) was observed as evident by Figure 2 (b). The acids first rapidly decreased the pH and then an increase was observed with the same speed back however due to the presence of Al2(SO4)3 linear stable pH range was managed to maintain as acidification of soil was probably maintained by slow proton release from Al2(SO4)3. An unusual trend was observed by the combination of H2O2 with acids i.e., expected drastic decrease in pH was not observed. This may be due to series of chemical reactions initiated in soil after addition of H2O2.
Results in combination with two chemicals for silt loam are shown in Figure 2 (c). Al2(SO4)3 with H2O2 showed decrease in pH initially but after thirty days soil attained its original pH. When Al2(SO4)3was used in the combination of acids a drastic decrease in pH was observed. H2SO4 decreased the pH but its effect was neutralized by the buffering system of the soil. It has been found that the texture of the soil determines the buffering capacity of the soil; namely, clay has more capacity to resist pH change than loamy soil has, followed by sand due to low buffering capacity of sandy soil, which is ultimately due to its low clay and organic matter content [30]. But addition of concentrated acid may damage the soil texture and cause unbalance in nutrient availability [24].
Treatments Applied in Combination of Three Chemicals to Sandy Clay Loam, Clay Loam and Silt Loam
Treatment was also applied in combination of three chemicals i.e. Al2(SO4)3, H2O2 and H2SO4 to sandy clay loam, clay loam and silt loam as shown in Figure 3. In case of sandy clay loam, first decrease was observed due to direct acidification and then low pH was sustained by the action of Al2(SO4)3. In the case of clay loam, drastic decrease in pH due to direct acidification was sustained due to presence of Al2(SO4)3 and an excellent linear pH of 1.5 was observed for one twenty days. For silt loam same trend with little variation was observed. This means optimization of quantities applied would result in the attaining of desired pH range for cultivation successfully. In the case of silt loam, when Al2(SO4)3 was used in combination with H2O2 and H2SO4 a drastic decrease in pH (i.e.1.0) after twenty five days was observed due to direct acidification and the low pH of 3 was sustained due to action of Al2(SO4)3.
It is concluded that Al2(SO4)3 has great pH lowering potential alone as well as in combinations due to slow release of protons from H2SO4 and conversion of S into H2SO4 by the action of microbes. Similarly, H2O2, HCl and H2SO4 are helpful in designing the suitable combinations with other chemicals for conditioning of pH of soil. And in combination with other chemicals H2O2 helped in maintaining its pH due to modification in the existing buffering system of the soil. The quick and the long term pH maintenance of the studied soils in binary combinations with addition of acids i.e. HCl and H2SO4 were accomplished. While the ternary combination was even found to be better than the binary Moreover, a correlation established between the buffering capacities of different textured soils reveal that soils have buffering capacities in order of clay loam>sandy clay loam>silt clay loam for the studied soils.
Acknowledgment
The authors appreciate the Mubashar Ahmed (Scientific officer, Potato research center Abbottabad, Pakistan) for assistance in soil analysis.
Funding
The authors did not receive funding from any source for this project.
Conflicts of Interest
The authors declare no conflicts of interest.