e-ISSN: 2319-9849
e-ISSN: 2319-9849
1Department of Applied Science, UIET, Kurukshetra University, Kurukshetra, Haryana, India
2Department of Chemistry, MMEC, Maharishi Markandeshwar University, Mullana, Ambala, Haryana, India
Received Date: 20/07/2016; Accepted Date: 14/10/2016; Published Date: 20/10/2016
Visit for more related articles at Research & Reviews: Journal of Chemistry
4H-1-benzopyrans and their derivatives have been used widely as analytical reagents for the spectrophotometric determination of several transition metal ions. The present article reviews the importance of 4H-1-benzopyrans as analytical or complexing or chelating agent and fundamental conditions of several methods used for analytical determination of metal ions spectrophotometrically.
4H-1-Benzopyrans, Transition metal ions, Extraction, Spectrophotometry, Determination
4H-1-benzopyrans and their derivatives constitute a versatile class of analytical/complexing/chelating reagents for the spectrophotometric determination of various transition metals.
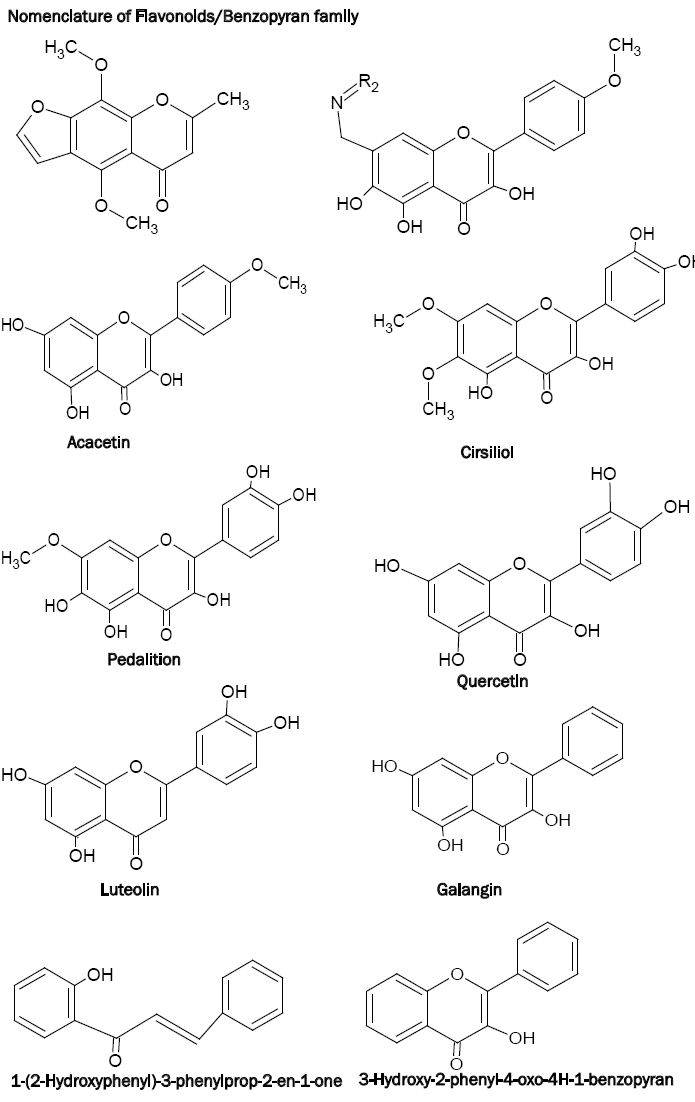
Benzopyrans are well known by the names chromones and flavonoids which play an important role in plants growth and development and in protection against microorganisms as well as pests [1]. Benzopyrans, in human diet, act as important antioxidants [2]. A large number of plant medicines contain benzopyrans having antibacterial, antimutagenic, antiviral, antineoplastic, antithrombotic and vasodilatory action [3-10]. For example, Khelin has been observed to possess coronary dilating action [11] whereas 7-hydroxy-8-dialkyl aminomethylflavones are observed to act as heart stimulants [12]. Benzopyrans have also been found to inhibit a wide range of enzymes involved in oxidation system such as 5-lipoxygenase, cyclooxygenase, monooxygenase or xanthine oxidase [13].
Horie et al. [10,14] have found that cirsiliol and pedalition act as potent 5-lipo oxygenase inhibitor whereas its synthetic analogues exhibit significant antiviral and antimicrobial activities. Some polyhydroxy flavones such as quercetin, luteolin, galangin etc. have been used as dyestuffs for many centuries.
The immediate family members of benzopyrans or flavonoids include flavones, flavonols, isoflavones and flavanones (2,3-dihydroxy derivative of flavones). Since the benzopyrans have been named in different ways, insertion of the following chart on the nomenclature of benzopyrans is necessary.
Benzopyrans containing a highly conjugated aromatic system, exhibit intense and characteristic absorption spectra in ethanol. Complexation or chelation of benzopyrans with various reagents leads to shifting in the spectra, thereby giving more information about the structure of benzopyrans [15]. As far as the analytical applications of benzopyrans are concerned, the flavonols such as morin, quercetin, galangin etc. have been used effectively. These chelating ligands possess oxygen donor carbonyl and the hydroxyl groups in their structures which is responsible for their analytical characteristics. The complexation of the 3-hydroxyl group and carbonyl group with metal resulting in a chelating structure is shown below
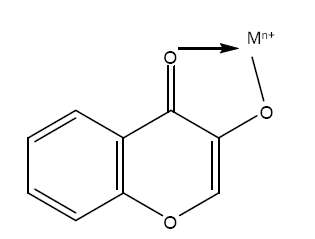
The present communication involves a review on the application of benzopyrans of compounds as chelating agents with various transition metal ions. The complexes formed were found to be highly sensitive and can be made more selective by maintaining various conditions (pH, solvent extraction and use of masking or complexing agents).
Thus, in search of new, better, sensitive and selective reagents, a detailed study of the 4H-1-benzopyrans and their derivatives containing oxygen donor carbonyl and the hydroxyl substituents has been carried out. The aim of this review is to summarize the analytical aspects of some selected benzopyrans.
During the last two decades, a large number of 4H-1-benzopyran derivatives possessing the chelating tendency with various transition metal ions have been synthesized. Some of them are summarized below.
3-hydroxy-2-(2'-thienyl)-4H-chromen-4-one (HTC)/3-hydroxy-2-(2'-thienyl)-4-oxo-4H-1-benzopyran (HTB)
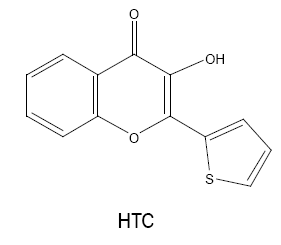
Chakkar and Kakkar [16] used HTC for spectrophotometric determination of niobium (V) for the first time. The reagent reacts with metal to form a chelating 1:2 coloured complex in perchloric acid medium. The complex is extractable into dichloromethane, quite stable, obeys Beer’s law in the range 0-1.9 μg Nb(V) ml-1 and shows molar absorptivity of 5.0357 × 104 l mol-1cm-1 at 420 nm.
HTC forms a 1:2 (M:L) dark yellow complex with vanadium (V) [17] in acetic acid medium. The complex is extractable into benzene and has λmax at 415-425 nm. The molar absorptivity and Beer's law obedience range of the formed complex are 3.27 × 104 l mol-1 cm-1 and 0-1.3 μg V(V) ml-1, respectively.
Agnihotri et al. [18] used the same reagent for the determination of V(III) in the analysis of various industrial samples including reverberatory flue dust and high speed steel super rapid extra 500. The 1:1 yellow complex formed between V(III) and HTC at 47ºC-57ºC from 0.03-0.14 mol l-1 acetic acid medium, is extractable into carbon tetrachloride and has λmax at 420 nm. Molar absorptivity and Beer's law range of the complex are 7.45 × 104 l mol-1 cm-1 and 0-0.85 μg V(III) ml-1, respectively. Presence of a large number of elements, anions and complexing agents do not interfere with the determination.
HTC is used by Nijhawan et al. for the determination of Zr (IV) under different conditions. At pH 8-8.2, a 1:3 complex is formed between Zr (IV) and HTC which is extractable into 1,2-dichloroethane showing λmax at 425 nm [19]. The complex obeys Beer's law in the Zr concentration range 0-1.0 μg ml-1 and has the ε value as 7.52 × 104 l mol-1 cm-1.
HTC [20] is again used for the trace determination of zirconium in HCl medium forming a yellow coloured complex which is made water soluble by miscellary action of Triton X-100 and measured at 415 nm. Beer's law range of the complex is 0-2.0 μg Zr(IV) ml-1 with ε value 2.73 × 104 l mol-1 cm-1.
Thorium (IV) [21] is determined spectrophotometrically as 1:4 yellow complex with HTC in dilute acetic acid medium. The complex shows molar absorptivity of 3.95 × 104 l mol-1 cm-1 at 430 nm.
HTC is used as a complexing agent for the determination of Mo(VI) by dissolving the complex formed in water in presence of Triton X-100 [22]. Molar absorptivity of the method is 2.80 × 105 l mol-1 cm-1 at 410 nm and Beer's law validity range is 0.01-0.4 μg Mo(VI) ml-1.
HTC/HTB is also used for the complexation of W(VI) [23] in trace amounts from 0.2 mol l-1 HCl solution. The dichloromethane extractable 1:2 (M:L) complex obeys Beer's law in the range of 0-2.8 μg W(VI) ml-1 and shows the molar absorptivity of 6.45 × 104 l mol-1 cm-1.
In alkaline medium, at a pH 8.5-9.2, Pd(II) [24] forms a 1:1 (M:L) yellow complex with HTC which is extractable into chloroform; the molar extinction coefficient being 3.301 × 104 l mol-1 cm-1, Beer's law range 0.01-0.1 μg Pd(II) ml-1 and λmax of the complex is at 455 nm.
In slightly acidic medium, a spectrophotometric method is developed for trace determination of Sn(II) [25] which involves the formation of a 1:2 yellow complex with HTC. The complex is extractable into dichloromethane, shows λmax at 432 nm and is stable upto two days. Beer's law is obeyed in the tin concentration range of 0-1.0 μg ml-1 and has molar absorptivity of 5.485 × 104 l mol-1 cm-1 at 432 nm. The method is successfully applied to the analysis of synthetic samples and alloy sample of gun metal.
Substituted HTC/HTB
In search for the new sensitive and selective reagents, a vast knowledge of different benzopyran derivatives has been gained as below
6-chloro-3-hydroxy-2-(2'-thienyl)-4-oxo-4H-1-benzopyran (CHTB):
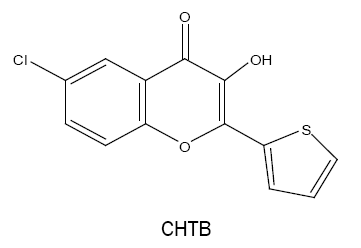
For the trace determination of tungsten(VI) in an acidic medium, an extractive spectrophotometric method is developed which involves the formation of a 1:3 complex of tungsten(VI) with CHTB [26] in 0.16-0.32 mol l-1 HCl medium. The complex is extractable into chloroform and shows maximum absorbance at 417-423 nm. The method obeys Beer's law in range 0-3.0 μg W(VI) ml-1 with molar absorptivity of 4.05 × 104 l mol-1 cm-1. This method is selective towards the analysis of tungsten in various synthetic, technical and natural samples including reverberatory flue dust and water.
The chelating ligand CHTB forms coloured metal chelates with Sn(II) [27] which is quantitatively extracted into dichloromethane with the molar extinction coefficient value greater than that of Sn(II)-HTB chelates.
6-chloro-3-hydroxy-7-methyl-2-(2'-thienyl)-4H-chromen-4-one (CHMTC):
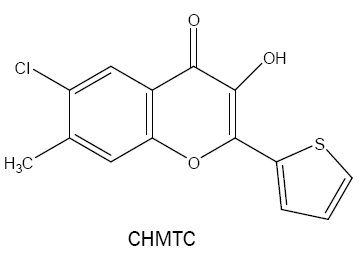
Agnihotri et al. [28] used another derivative of HTC named as CHMTC as a very strong complexing agent for vanadium(V) in weakly acidic (HCl; pH 0.84-1.09) medium. The method developed is found to be highly sensitive with considerably greater molar absorptivity of 8.26 × 104 l mol-1 cm-1 and selective binary system for the extractive photometric determination of vanadium.
The same reagent is used for the spectrophotometric dewtermination of Zr (IV) in HCl medium [29]. The 1:3 (M:L) complex is extractable into benzene, shows maximum absorbance at 430 nm with the molar absorbptivity of 5.6 × 104 l mol-1 cm-1.
3-hydroxy-2-(3'-methyl-2'-thienyl)-4-oxo-4H-1-benzopyran (HMTB):
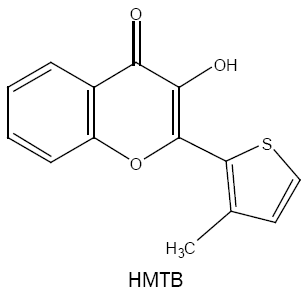
Using HMTB, a simple, sensitive and selective approach is developed for the analysis of tungsten(VI) in several samples of varying complexity [30]. The coloured species has molar absorptivity of 5.80 × 104 l mol-1 cm-1 at 420 nm and the method is free from the interference of a number of analytically important elements.
2-(2-furyl)-3-hydroxy-4H-chromen-4-one (FHC) /2-(2'-furyl)-3-hydroxy-4-oxo-4H-1-benzopyran (FHB)
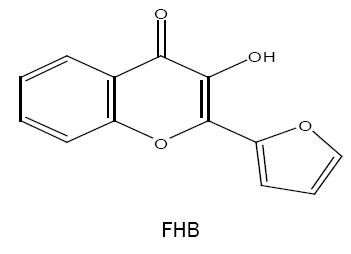
Analytical properties of 2-(2'-furyl)-3-hydroxy-4-oxo-4H-1-benzopyran (FHB) and simultaneous spectrophotometric determination of molybdenum (V,VI) [31,32] are reported by Mehta et al. The methods are applied successfully for the determination of metal ions in various synthetic and technical sample including steels and flue dust.
FHC/FHB is recommended as a chelating reagent by Ghai et al. [33], where it forms 1:2 yellow coloured chelating complex with vanadium (V) in acetic acid medium. The complex is extractable into chloroform, has λmax at 410 nm and shows stability for more than 18 hrs. The molar extinction coefficient (ε) of the complex is 2.24 × 104 l mol-1 cm-1. Sn(II), Mo(VI), ascorbic acid, oxalate and disodium 'EDTA' interfere during the determination. However, the method is simple, rapid and has been satisfactorily applied for the determination of vanadium in synthetic and technical samples.
Extraction characteristic and analytical behaviour of W(VI), Nb(V) and Zr(IV) [34-36] chelates with FHC are also studied by Agnihotri and Mehta. The same reagent that is FHC is used by Kataria et al. for the determination of Sn(II) in various synthetic and technical samples [37].
Substituted FHC/FHB
6-chloro-2-(2'-furyl)-3-hydroxy-4H-chromen-4-one (CFHC):
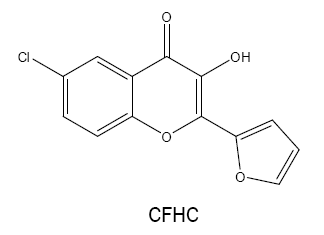
The possibility of CFHC as an analytical reagent has been described by using vanadium(III) [38]. When sodium dithionite is employed as a reductant, Vanadium(V) is reduced to Vanadium(III) which forms a 1:3 yellow coloured complex with CFHC in acidic medium at 50-60ºC. The coloured species formed is quantitatively extracted into benzene with the absorbance measured at 415- 425 nm. The molar extinction coefficient is found to be greater than that of the V(V) complex with FHC alone.
CHFC has been applied as a ligand to complex with the analyte metal ion W(VI) [39]. The light yellow complex is extracted into dichloromethane from HCl solution and has a molar absorptivity of 2.2 × 104 l mol-1 cm-1. Nb(V) also reacts with CFHC in aqueous solution. The complex can be extracted into chloroform from 4 mol l-1 HClO4 solution [40]. The molar extinction coefficient in the extract is 2.79 × 104 l mol-1 cm-1 at 410 nm.
6-chloro-3-hydroxy-2-(2'-(5'-methylfuryl)-4H-chromen-4-one (CHMFC):
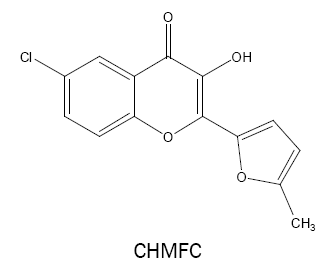
CHMFC is found to react with vanadium(V) in acetic acid medium to form a 1:1 dark yellow complex (ε =3.98 × 104 l mol-1 cm-1, λmax =432 nm) [41] which has been used for the determination of vanadium in synthetic and technical samples including steel, flue dust and water samples.
The chelating behaviour of CHMFC is studied on complexation with W(VI) [42]. Bishnoi et al. [43] studied the reaction between CHMFC and Mo(VI). A 1:2 yellow complex is formed in acetic acid medium having molar absorptivity of 5.36 × 104 l mol-1 cm-1.
Other benzopyran compounds as analytical reagents
3-hydroxy-2-phenyl-4-oxo-4H-1-benzopyran (HPB) and derivative:
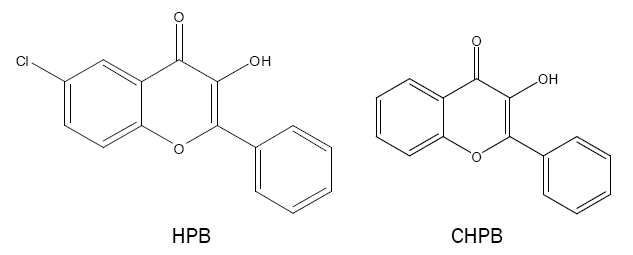
HPB and its derivative such as 6-chloro-3-hydroxy-2-phenyl-4-oxo-4H-1-benzopyran (CHPB) are synthesised and the compounds are used as analytical reagents for the spectrophotometric determination of Sn(II) [44,45] and W(VI) [46,47]. The reagents show extreme sensitivity, selectivity and colour stability in the reaction in acidic medium.
3-hydroxy-2-(4'-methoxyphenyl)-4-oxo-4H-1-benzopyran (HMPB):
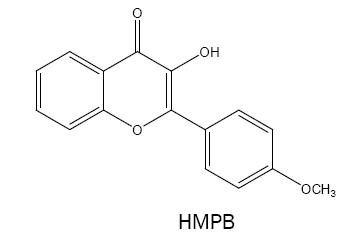
HMPB have been used as a chromogenic reagent for spectrophotometric determination of Nb(V) [48] and W(VI) [49]. Its methyl derivative i.e., 3-hydroxy-2-(4'-methylphenyl)-6-methyl-4H-chromen-4-one has attracted more attention as a spectrophotometric reagent for complexation with V(V,III) [50,51], W(VI) [52] and Mo(VI) [53]. On the other hand, the 6-chloro derivative i.e., 6-chloro-3- hydroxy-2-(4'-methoxyphenyl)-4-oxo-4H-1-benzopyran is a useful and sensitive reagent for W(VI) [54].
Sn(II) reacts with another derivative of HMPB i.e., 6-chloro-3-hydroxy-7-methyl-2-(4'-methoxyphenyl)-4-oxo-4H-1-benzopyran [55] to form a yellow coloured complex in HCl medium which is extracted into dichloromethane and shows λmax at 415 nm. The molar absorptivity of the complex is enhanced to a value of 6.45 × 104 l mol-1 cm-1.
Dass et al. [56,57] have studied the reaction behaviour of Mo (VI, V) with the other derivative like 3-hydroxy-2-(4'-methoxyphenyl)- 6-propionyl-4H-chromen-4-one.
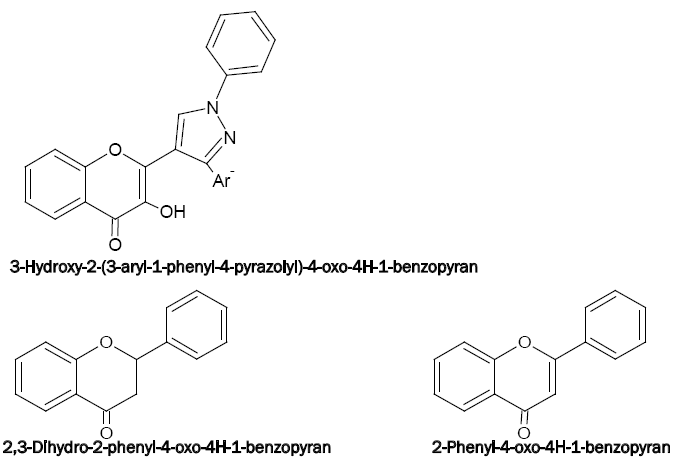
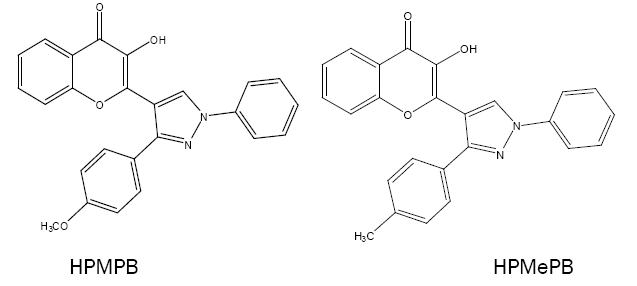
Some new pyrazolyl derivatives of 4H-1-benzopyran are recently synthesised as 3-hydroxy-2-[1'-phenyl-3'-(p-methylphenyl)- 4'-pyrazolyl]-4-oxo-4H-1-benzopyrans(HPMPB),3-hydroxy-2-[1'-phenyl-3'-(4'-methoxyphenyl)-4'-pyrazolyl]-4-oxo-4H-1-benzopyran (HPMePB) and 3-hydroxy-2-[1'-phenyl-3'-(p-chlorophenyl)-4'-pyrazolyl]-4-oxo-4H-1-benzopyran (HPCPB). These act as chomogenic reagents for the spectrophotometric determination of V(V) (with HPMPB) [58], Sn(II) (with HPMePB) [59] and V(V), W(VI) and Nb(V) (with HPMPB) [60-62], respectively.
The applications of 4H-1-benzopyrans in the separation and determination of metal ions by using spectrophotometer are presented in Table 1 [44-62]. For every derivative, the conditions for separation and determination of corresponding metal ions (medium, λmax , Beer's law range, molar extinction coefficient (ε), metal to ligand stoichiometry (M:L) and the solvent ) are summarized. The summary clearly indicates the promising complexing agent nature of 4H-1-benzopyrans.
| S. No. | Metal ion |
Reagent | λmaxnm (Solvent) | Medium | Beerʹs Law range, µg ml-1 | ε × 104 | Stoichio-metry (M:L) | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | Sn(II) | 3-hydroxy-2-phenyl-4-oxo-4H-1-benzopyran | 405 (dichloromethane) |
HCl | 0-2.0 | 5.46 | 01:02 | 43 |
| 2 | Sn(II) | 6-chloro-3-hydroxy-2-phenyl-4-oxo-4H-1-benzopyran | 415 (carbon tetrachloride) | HCl | 0-2.0 | 3.92 | 01:02 | 44 |
| 3 | W(VI) | 3-hydroxy-2-phenyl-4-oxo-4H-1-benzopyran | 391 (dichloromethane) | HCL | 0-2.7 | 4.29 | 01:02 | 45 |
| 4 | W(VI) | 6-chloro-3-hydroxy-2-phenyl-4-oxo-4H-1-benzopyran | 420 (chloroform) | HCl | 0-2.9 | 3.13 | 01:02 | 46 |
| 5 | Nb(V) | 3-hydroxy-2-(4ʹ-methoxyphenyl)-4-oxo-4H-1-benzopyran | 405 (dichloromethane) | HClO4 | 0-1.3 | 3.76 | 01:03 | 47 |
| 6 | W(VI) | 3-hydroxy-2-(4ʹ-methoxyphenyl)-4-oxo-4H-1-benzopyran | 407 (dichloromethane) | HClO4 | 0-3.8 | 3.78 | 01:04 | 48 |
| 7 | V(V) | 3-hydroxy-2-(4ʹ-methoxyphenyl)-6-methyl-4-oxo-4H-1-benzopyran | 420 (benzene) | CH3COOH | 0-1.3 | 3.27 | 01:02 | 49 |
| 8 | V(III) | 3-hydroxy-2-(4ʹ-methoxyphenyl)-6-methyl-4-oxo-4H-1-benzopyran | 410 (chloroform) | CH3COOH | 0-2.0 | 3.3 | 01:03 | 50 |
| 9 | W(VI) | 3-hydroxy-2-(4ʹ-methoxyphenyl)-6-methyl-4-oxo-4H-1-benzopyran | 410 (dichloromethane) | HClO4 | 0-3.1 | 4.69 | 01:02 | 51 |
| 10 | Mo(VI) | 3-hydroxy-2-(4ʹ-methoxyphenyl)-6-methyl-4-oxo-4H-1-benzopyran | 411 (1,2-dichloroethane) | CH3COOH | 0-2.3 | 5.61 | 01:02 | 52 |
| 11 | W(VI) | 6-chloro-3-hydroxy-2-(4ʹ-methoxyphenyl)-4-oxo-4H-1-benzopyran | 415 (dichloromehane) | HCl | 0-2.1 | 6.08 | 01:02 | 53 |
| 12 | Sn(II) | 6-chloro-3-hydroxy-7-methyl-2-(4ʹ-methoxyphenyl)-4-oxo-4H-1-benzopyran | 426 (dichloromethane) | HCl | 0-1.68 | 6.29 | 01:02 | 54 |
| 13 | Mo(VI) | 3-hydroxy-2-(4ʹ-methoxyphenyl)-6-propionyl-4-oxo-4H-1-benzopyran | 416 (1,2-dichloroethane) | CH3COOH | 0-2.5 | 5.56 | 01:02 | 55 |
| 14 | Mo(V) | 3-hydroxy-2-(4ʹ-methoxyphenyl)-6-propionyl-4-oxo-4H-1-benzopyran | 414 (1,2-dichloroethane) | H2SO4 | 0-2.45 | 5.43 | 01:02 | 56 |
| 15 | V(V) | 3-hydroxy-2-[1ʹ-phenyl-3ʹ-(p-methylphenyl)-4ʹ-pyrazolyl]-4-oxo-4H-1-benzopyran | 415 (toluene) | CH3COOH | 0-1.2 | 2.5 | 01:03 | 57 |
| 16 | Sn(II) | 3-hydroxy-2-[1ʹ-phenyl-3ʹ-(4ʺ-methoxyphenyl)-4ʹ-pyrazolyl]-4-oxo-4H-1-benzopyran | 424 (carbon tetrachloride) | HCl | 0-3.04 | 4.87 | 01:02 | 58 |
| 17 | V(V) | 3-hydroxy-2-[1ʹ-phenyl-3ʹ-(p-chlorophenyl)-4ʹ-pyrazolyl]-4-oxo-4H-1-benzopyran | 410 (carbon tetrachloride) | CH3COOH | 0-1.4 | 2.2 | 01:03 | 59 |
| 18 | W(VI) | 3-hydroxy-2-[1ʹ-phenyl-3ʹ-(p-chlorophenyl)-4ʹ-pyrazolyl]-4-oxo-4H-1-benzopyran | 405 (chloroform) | HClO4 | 0-1.3 | 5.71 | 01:04 | 60 |
| 19 | Nb(V) | 3-hydroxy-2-[1ʹ-phenyl-3ʹ-(p-chlorophenyl)-4ʹ-pyrazolyl]-4-oxo-4H-1-benzopyran | 415 (chloroform) | HClO4 | 0-1.2 | 2.79 | 01:04 | 61 |
| 21 | Mo(VI) | 3-hydroxy-2-(2’-thienyl)-4-oxo-4H-1-benzopyran | 410 (Triton X-100) | H2O | 0-0.4 | 2.8 | - | 62 |
Table 1: Applications of 4H-1-benzopyrans as Analytical Reagents
This review article gives an updated survey of large number of 4H-1-benzopyran derivatives and their importance towards chelation of various transition metals. From the review, we revealed that 4H-1-benzopyrans are good chromogenic reagents for the analytical determination of metal ions due to their strongly chelating tendency. 4H-1-benzopyrans can be employed for the spectrophotometric determination of metal ions such as V(V,VI), Mo(V,VI), Nb(V), W(VI), Zr(IV), Th(IV), Ce(IV), Sn(II) and Pd(II). So, it is strongly believed that the present review article will be beneficial to the analytical chemists working in the same area of interest.
Authors sincere thanks are due to the Authorities, Maharishi Markandeshwar University, Mullana for providing the necessary and desired facilities.