e-ISSN: 2320-0812
e-ISSN: 2320-0812
Sneha Lakshmi R.P*
Department of Pharmaceutical Analysis, Omega College of Pharmacy, Osmania University, Hyderabad, India
Received date: 03 March 2015; Accepted date: 26 March 2015; Published date: 31 March 2015
Visit for more related articles at Research & Reviews: Journal of Pharmaceutical Analysis
The proposed method was found to be simple, precise, accurate and rapid for determination of Metadoxine and Atazanavir from pure and its dosage forms. The mobile phase is simple to prepare and economical. The sample recoveries in all formulations were in good agreement with their respective label claims and they suggested non-interference of formulation excipients in the estimation. Hence, this method can be easily and conveniently adopted for routine analysis of Metadoxine and Atazanavir in pure form and its dosage forms and can also be used for dissolution or similar studies.
Chromatography, Pharmacodynamic agents, Chemotherapeutic agents
A drug may be defined as a substance meant for diagnosis, cure, mitigation, prevention or treatment of diseases in human beings or animals or for alternating any structure or function of the body of human being or animals. Pharmaceutical chemistry is a science that makes use of general laws of chemistry to study drugs i.e. their preparation, chemical nature, composition, structure, influence on an organism and studies the physical and chemical properties of drugs, the methods of quality control and the conditions of their storage etc. The family of drugs may be broadly classified as,
1. Pharmacodynamics agents
It is necessary to find the content of each drug either in pure or single, combined dosage forms for purity testing. It is also essential to know the concentration of the drug and it’s metabolites in biological fluids after taking the dosage form for treatment. The scope of developing and validating analytical methods is to ensure a suitable method for a particular analyte more specific, accurate and precise. The main objective for that is to improve the conditions and parameters, which should be followed in the development and validation.
A survey of literature reveals that good analytical methods are not available for the drugs like Metadoxine, and Atazanavir. Even though very few methods of estimation of above drugs are available, many of them suffer from one disadvantage or the other, such as low sensitivity, lack of selectivity and simplicity etc. The existing physicochemical methods are inadequate to meet the requirements, hence it is proposed to improve the existing methods and to develop new methods for the assay of Metadoxine, and Atazanavir in pharmaceutical dosage forms adapting different available analytical techniques like UV-Spectrophotometry, Chromatography and HPLC. [1-10]
The term ‘Chromatography’ covers those processes aimed at the separation of the various species of a mixture on the basis of their distribution characteristics between a stationary and a mobile phase.
Modes of chromatography are defined essentially according to the nature of the interactions between the solute and the stationary phase, which may arise from hydrogen bonding, Vander walls forces, electrostatic forces or hydrophobic forces or basing on the size of the particles (e.g. Size exclusion chromatography). Different modes of chromatography are as follows: [11-15]
• Normal Phase Chromatography
• Reversed Phase Chromatography
• Reversed Phase – ion pair Chromatography
• Size Exclusion Chromatography
In normal phase chromatography, the stationary phase is a polar adsorbent and the mobile phase is generally a mixture of non-aqueous solvents.
The silica structure is saturated with silanol groups at the end. These OH groups are statistically disturbed over the whole of the surface. The silanol groups represent the active sites (very polar) in the stationary phase. This forms a weak type of bond with any molecule in the vicinity when any of the following interactions are present
• Dipole-induced dipole
• Dipole-dipole
• Hydrogen bonding
• π-Complex bonding
These situations arise when the molecule has one or several atoms with lone pair electron or a double bond. The adsorption strengths and hence k’ values (elution series) increase in the following order. Saturated hydrocarbon < olefins < aromatics < organic halogen compounds < sulphides < ethers < esters < aldehydes and ketones < amines < sulphones < amides < carboxylic acids. The strength of interactions depends not only on the functional groups in the sample molecule but also on steric factors. If a molecule has several functional groups, then the most polar one determines the reaction properties. [15-20]
Chemically modified silica, such as the aminopropyl, cyanopropyl and diol phases is useful alternatives to silica gel as stationary phase in normal phase chromatography. [21]
The aminopropyl and cyanopropyl phases provide opportunities for specific interactions between the analyte and the stationary phases and thus offer additional options for the optimizations of separations. Other advantages of bonded phases lie in their increased homogeneity of the phase surface. [22]
Resolution with water in weak mobile phase may be most conveniently achieved by drying the solvents and then adding a constant concentration of water or some very polar modifier such as acetic acid or triethylamine (TEA) to the mobile phase. The addition of such polar modifiers serves to deactivate the more polar shape as well as the reproducibility of the retention times. [23,24]
In 1960’s chromatographers started modifying the polar nature of silanol group by chemically reacting silica with organic silanes, the objective was to make less polar or non-polar so that polar solvents can be used to separate water-soluble polar compounds. Since the ionic nature of the chemically modified silica is now reversed i.e. it is non-polar or the nature of the phase is reversed. The chromatographic separation carried out with such silica is referred to as reversed- phase chromatography. [25-30]
A large number of chemically bonded stationary phases based on silica are available commercially. Silica based stationary phases are still most popular in reversed phase chromatography however other adsorbants based on polymer (styrene-divinyl benzene copolymer) are slowly gaining ground. [31,32]
Simple compounds are better retained by the reversed phase surface, the less water- soluble (i.e. the more non-polar) they are. The retention decreases in the following order: aliphatics > induced dipoles (i.e. CCl4) > permanent dipoles (e.g.CHCl3) > weak lewis bases (ethers, aldehydes, ketones) > strong lewis bases (amines) > weak lewis acids (alcohols, phenols) > strong lewis acids (carboxylic acids). Also the retention increases as the number of carbon atoms increases. [33,34]
As a general rule the retention increases with increasing contact area between sample molecule and stationary phase i.e. with increasing number of water molecules, which are released during the adsorption of a compound. Branched chain compounds are eluted more rapidly than their corresponding normal isomers. [35,36]
Chemically bonded octadecyl silane (ODS) an alkaline with 18 carbon atoms it is the most popular stationary phase used in pharmaceutical industry. Since most pharmaceutical compounds are polar and water soluble, the majority of HPLC methods used for quality assurance, decomposition studies, quantitative analysis of both bulk drugs and their formulations use ODS HPLC columns. The solvent strength in reversed phase chromatography is reversed from that of adsorption chromatography (silica gel) as stated earlier. Water interacts strongly with silanol groups, so that, adsorption of sample molecules become highly restricted and they are rapidly eluted as a result. Exactly opposite applies in reversed phase system; water cannot wet the non-polar (hydrophobic) alkyl groups such as C18 of ODS phase and therefore does not interact with the bonded moiety. Hence water is the weakest solvent of all and gives slowest elution rate. The elution time (retention time) in reversed phase chromatography increases with increasing amount of water in the mobile phase. [37-40]
Chromatographic methods can be classified most practically according to the stationary and mobile phases, as shown in the table 1.
The importance of Chromatography is increasing rapidly in pharmaceutical analysis. The exact differentiation, selective identification and quantitative determination of structurally closely related compounds can be performed by chromatography. Another important field of application of chromatographic methods is the purity testing of final products and intermediates (detection of decomposition products and by-products). As a consequence of the above points, chromatographic methods are occupying an ever-expanding position in the latest editions of the pharmacopoeias and other testing standards. The modern form of column chromatography has been called high performance, high pressure, and high-resolution and high-speed liquid chromatography. [41-45]
High-Performance Liquid Chromatography (HPLC) is a special branch of column chromatography in which the mobile phase is forced through the column at high speed. As a result the analysis time is reduced by 1-2 orders of magnitude relative to classical column chromatography and the use of much smaller particles of the adsorbent or support becomes possible increasing the column efficiency substantially. [42]
The essential equipment consists of an eluent, reservoir, a high-pressure pump, and an injector for introducing the sample, a column containing the stationary phase, a detector and recorder. The development of highly efficient micro particulate bonded phases has increased the versatility of the technique and has greatly improved the analysis of multi component mixtures. [43]
The systems used are often described as belonging to one of four mechanistic types, adsorption, partition, ion exchange and size-exclusion. Adsorption chromatography arises from interaction between solutes on the surface of the solid stationary phase. Partition chromatography involves a liquid stationary phase, which is immiscible with the eluent and coated on an inert support. Adsorption and partition systems can be normal phase (stationary phase more polar than eluent) or reversed phase (stationary phase less polar than eluent). Ion-exchange chromatography involves a solid stationary phase with anionic or cationic groups on the surface to which solute molecules of opposite charge are attracted. Size-exclusion chromatography involves a solid stationary phase with controlled pore size. Solutes are separated according to their molecular size, the large molecules enable to enter the pores eluting first. [44]
The modern form of column chromatography has been called high performance, high pressure, and high-resolution and high-speed liquid chromatography. [45]
The mobile phase is pumped under pressure from one or several reservoirs and flows through the column at a constant rate. With micro particulate packing, there is a high-pressure drop across a chromatography column. Eluting power of the mobile phase is determined by its overall polarity, the polarity of the stationary phase and the nature of the sample components. For normal phase separations eluting power increases with increasing polarity of the solvent but for reversed phase separations, eluting power decreases with increasing solvent polarity. Optimum separating conditions can be achieved by making use of mixture of two solvents. Some other properties of the solvents, which need to be considered for a successful separation, are boiling point, viscosity, detector compatibility, flammability and toxicity. [46]
The most important component of HPLC in solvent delivery system is the pump, because its performance directly effects the retention time, reproducibility and detector sensitivity. Among the several solvent delivery systems (direct gas pressure, pneumatic intensifier, reciprocating etc.) reciprocating pump with twin or triple pistons is widely used, as this system gives less baseline noise, good flow rate reproducibility etc. [47]
The constituents of the mobile phase should be degassed and filtered before use. Several methods are employed to remove the dissolved gases in the mobile phase. They include heating and stirring, vacuum degassing with an aspirator, filtration through 0.45 filters, vacuum degassing with an air-soluble membrane, helium purging ultra-sonication or purging or combination of these methods. HPLC systems are also provided an online degassing system, which continuously removes the dissolved gases from the mobile phase. [48-50]
HPLC columns may be run isocratically, i.e., with constant eluent or they may be run in the gradient elution mode in which the mobile phase composition varies during run. Gradient elution is a means of overcoming the problem of dealing with a complex mixture of solutes. [51]
Two means for analyte introduction on the column are injection in to a flowing stream and a stop flow injection. These techniques can be used with a syringe or an injection valve. Automatic injector is a microprocessor-controlled version of the manual universal injector. Usually, up to 100 samples can be loaded in to the auto injector tray. The system parameters such as flow rates, gradient, run time, volume to be injected, etc. are chosen, stored in memory and sequentially executed on consecutive injections. [52-54]
The function of the detector in HPLC is to monitor the mobile phase as it emerges from the column. Generally, there are two types of HPLC detectors, bulk property detectors and solute property detectors. [55,56]
These detectors are based on differential measurement of a property, which is common to both the sample and the mobile phase. Examples of such detectors are refractive index, conductivity and dielectric constant detectors. [57-60]
Solute property detectors respond to a physical property of the solute, which is not exhibited by the pure mobile phase. These detectors measure a property, which is specific to the sample, either with or without the removal of the mobile phase prior to the detection. Solute property detectors which do not require the removal of the mobile phase before detection include spectrophotometric (UV or UV-Vis) detector, fluorescence detectors, polarographic, electro-chemical and radio activity detectors, whilst the moving wire flame ionization detector and electron capture detector both require removal of the mobile phase before detection.
UV-Vis and fluorescent detectors are suitable for gradient elution, because many solvents used in HPLC do not absorb to any significant extent. [61,62]
The heart of the system is the column. In order to achieve high efficiency of separation, the column material (micro-particles, 5-10 μm size) packed in such a way that highest numbers of theoretical plates are possible. Silica (SiO2, H2O) is the most widely used substance for the manufacture of packing materials. It consists of a network of siloxane linkages (Si-O-Si) in a rigid three dimensional structure containing inter connecting pores. Thus a wide range of commercial products is available with surface areas ranging from 100 to 800 m2/g. and particle sizes from 3 to 50 μm. [63-65]
The silanol groups on the surface of silica give it a polar character, which is exploited in adsorption chromatography using non-polar organic eluents. Silica can be drastically altered by reaction with organo chloro silanes or organo alkoxy silanes giving Si-O-Si-R linkages with the surface. The attachment of hydrocarbon change to silica produces a non-polar surface suitable for reversed phase chromatography where mixtures of water and organic solvents are used as eluents. The most popular material is octadecyl-silica (ODS-Silica), which contains C18 chains, but materials with C2, C6, C8 and C22 chains are also available. During manufacture, such materials may be reacted with a small mono functional silane (e.g. trimethyl chloro silane) to reduce further the number of silanol groups remaining on the surface (end-capping). There is a vast range of materials which have intermediate surface polarities arising from the bonding to silica of other organic compounds which contain groups such as phenyl, nitro, amino and hydroxyl. Strong ion exchangers are also available in which sulphonic acid groups or quaternary ammonium groups are bonded to silica. The useful pH range for columns is 2 to 8, since siloxane linkages are cleaved below pH-2 while at pH values above eight silica may dissolve. [66,67]
In HPLC, generally two types of columns are used, normal phase columns and reversed phase columns. Using normal phase chromatography, particularly of non-polar and moderately polar drugs can make excellent separation. It was originally believed that separation of compounds in mixture takes place slowly by differential adsorption on a stationary silica phase. [68-70]
However, it now seems that partition plays an important role, with the compounds interacting with the polar silanol groups on the silica or with bound water molecules. [71,72]
While normal phase seems the passage of a relatively non-polar mobile phase over a polar stationary phase, reversed phase chromatography is carried out using a polar mobile phase such as methanol, Acetonitrile, water, buffers etc., over a non-polar stationary phase. Ranges of stationary phases (C18, C8, -NH2, -CN, -phenyl etc.) are available and very selective separations can be achieved. The pH of the mobile phase can be adjusted to suppress the ionization of the drug and thereby increase the retention on the column. For highly ionized drugs ion-pair chromatography is used. [73,74]
In HPLC derivatization is used to enhance the sensitivity and selectivity of detection when available detectors are not satisfactory for the underivatized compounds. Both ultra violet absorbing and fluorescence derivatives have been widely used. Ultra violet derivatization reagents include N-succinimidyl p-nitro phenyl acetate, phenyl hydrazine and 3, 5-dinitro benzyl chlorides, while fluorescent derivatives can be formed with reagents such as dansyl chloride, 4-bromo methyl-7-methoxy-coumarin and fluorescamine. Derivative formation can be carried out before the sample is injected on to the column or by online chemical reactions between the column out let and the detector. [75-80]
Gradient elution or solvent programming is the change of solvent composition during a separation in which the solvent strength increases from the beginning to the end of the separation. It is well suited to the analysis of samples of unknown complexity since good resolution is automatically provided for a wide range of sample polarities. There are two types of gradient systems: Low-pressure gradient mixtures and high- pressure gradient mixtures. In the former the solvents are mixed at atmosphere pressure and then pumped to the column, whereas in the later, solvents are pumped in to a mixing chamber at high pressure before going in to the column. [81]
System suitability
The system suitability test represents an integral part of the method and is used to ensure the adequate performance of the chosen chromatographic system.
Efficiency, capacity factor resolution factor, and symmetry factor are the parameters that are normally used in assessing the column performance. Factors that can affect chromatographic behavior include mobile phase composition, temperature, ionic strength, apparent pH, flow rate and column length and stationary phase characteristics such as porosity, particle size and type, and specific surface area. [82,83]
The efficiency of a chromatographic column is defined in terms of the number of theoretical plates (N) and can be calculated using the following formula: [84]
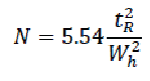
Where,
tR = retention time or the baseline distance between the point of injection and the perpendicular dropped from the maximum of the peak of interest.
Wh = the width of the peak of interest determined at half peak height, measured in the same units as tR.
N = the number of theoretical plates per meters.
The column plate number increases with several factors:
1. Well-packed columns (column “quality”)
2. Longer columns
3. Lower flow rates (but not too low)
4. Smaller column-packing particles
5. Lower mobile-phase viscosity and higher temperature
6. Smaller sample molecules
This factor determines the retention of a solute and can be calculated from the chromatogram using the following formula:
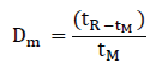
Where,
tR = retention time of the solute
tM = retention time of an unretained component
A low Dm value indicates that the peak elutes close to the solvent front, which may compromise selectivity. A minimum Dm value of 1 is recommended for the peak of interest.
The retention time of the test substance can be varied, if necessary, by changing the relative proportion or composition of solvents in the mobile phase. Generally, an increase in the proportion of a more polar solvent will lead to a shorter retention time on a normal-phase column and a longer retention time on a reversed-phase column. [85]
It is measure of the extent of separation of two compounds and the baseline separation is achieved. The resolution between two peaks of similar height in a chromatogram can be calculated using the following formula:
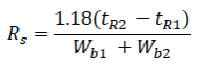
Where,
tR1 and tR2 = retention times or baseline distances between the point of injection and the perpendicular dropped from the maximum of each of the two peaks.
Wb1 and Wb2 = the respective peak widths determined at half peak height, measured in the same units as tR1 and tR2.
The value of Rs for a baseline separation between peaks of similar height should be at least. [86,87]
The relative retention (r) is calculated as an estimate using the following formula:
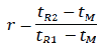
Where,
tR2 = retention time of the peak of interest
tR1 = retention time of the reference peak
tM = retention time of an unretained component
Retention time is the difference in time between the time of injection of a solute and time of elution of the peak maximum of that solute. Retention time is measured in minutes or seconds. Retention time is also proportional to the distance moved on a chart paper, which can be measured in cm or mm [88-90]
Retention volume is the volume of mobilephase required to elute 50% of the component from the column. It is the product of retention time and flow rate. [91]
Retention volume (Vr) = Retention time (Rt) x flow rate
It is called as the number of theoretical plates. It measures the band spreading of a peak. When band spread in smaller, the number of theoretical plates is higher. It indicates a good column and system performance. [92]
N=16 ( tR / W)2
A theoretical plate can be of any height, which decides the efficiency of separation. If HETP is less the column is more efficient. If HETP is more, the column is less efficient. The height equivalent to a theoretical plate (HETP) is given by
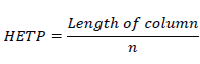
Where n = theoretical plates.
T = retention time of the components.
W=width of the base of the component peak using tangent method.
L= column length in meters
N=plates per meter
Symmetry factor (As)
The symmetry factor for a peak can be calculated using the following formula:
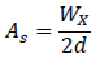
Where,
Wx = width at 5% of peak height measured from the baseline.
d = baseline distance between the perpendicular dropped from the peak maximum and the leading edge of the peak at 5% of the peak height, measured in the same units as Wx.
Values of as which are greater than 2 may lead to incorrect integration, resulting in erroneous quantitation. The main factors that influence peak symmetry depend upon retention, solvent effects, incompatibility of the solute with the mobile phase or development of an excessive void at the inlet of the column. In reversed-phase chromatography, adsorption phenomena due to the presence of residual silanol groups in the stationary phase may lead to tailing (poor peak symmetry). [93,94]
The Tailing Factor T, a measure of peak symmetry is unity for perfectly symmetrical peaks and its value increases as tailing becomes more pronounced. In some cases, values less than 1 may be observed. As peak asymmetry increases integration and hence precision becomes less reliable. [95,96]
Where,
W0.05 = width of peak at 5% height
f = Distance from the peak maximum to the leading edge of the peak, the distance being measured at a point 5% of the peak height from the baseline.
HPLC is an assertive analytical technique with sophisticated technologies that have been extensively practiced from decades. Modernizations such as ultrahigh-pressure liquid chromatography, liquid chromatography-mass spectrometry, two-dimensional liquid chromatography, chiral separations, core-shell columns, and novel stationary phases have helped drive HPLC to higher performance in diverse factors, yielding faster speed, higher resolution, greater sensitivity, and increased precision.
The practice of HPLC is bygone limited to analyzers, but is now widely performed by students, chemists, biologists, production workers, and other novices in academia, research, and quality control laboratories.