Keywords
|
| Infra Red (IR), Non-Dispersive Infra Red (NDIR), Gas Chromatograph (GC), support-coated open tubular (SCOT), wall-coated open tubular (WCOT) |
INTRODUCTION
|
| Gas analyzer is an instrument which is capable of analyzing the species of chemical gases is present in the sample. Not only it identifies the species but it also has capability to give measurement value of the quantity which it displays either in numerical form or shows it graphically. Depending on the type of principle selected for the gas analysis, it can be classified as follows: |
| A. Gas chromatograph |
| B. IR Gas analyzer |
| C. Thermal conductivity gas analyzer |
| D. Paramagnetic gas analyzer |
| E. Electrochemical analyzer |
| F. ORSAT apparatus |
| G. Gravimetry gas analyzer |
| H. Methanometer |
TYPES OF GAS ANALYZER
|
| There are various types of gas analyzer with their different working principle. Also due to their different principle of operation they are capable of estimating different species of gas. |
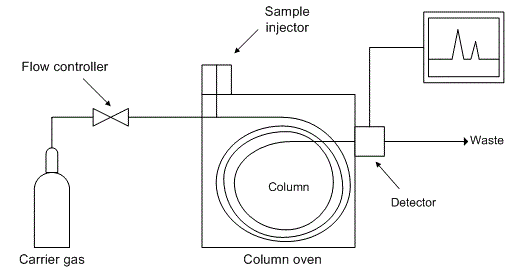
| A. Gas chromatograph: Gas chromatography involves a sample injected onto the head of the chromatographic column. The sample is transported through the column by the flow of inert, gaseous mobile phase. The column itself contains a liquid stationary phase which is adsorbed onto the surface of an inert solid. |
| Instrumental components |
| Carrier gas: The carrier gas must be chemically inert. Commonly used gases include nitrogen, helium, argon, and carbon dioxide. The choice of carrier gas is often dependent upon the type of detector which is used. The carrier gas system also contains a molecular sieve to remove water and other impurities. |
| Sample injection port: For optimum column efficiency, the sample should not be too large, and should be introduced onto the column as a "plug" of vapour - slow injection of large samples causes band broadening and loss of resolution. The most common injection method is where a microsyringe is used to inject sample through a rubber septum into a flash vapouriser port at the head of the column |
| Columns: There are two general types of column, packed and capillary (also known as open tubular). Packed columns contain a finely divided, inert, solid support material (commonly based on diatomaceous earth) coated with liquid stationary phase. Most packed columns are 1.5 - 10m in length and have an internal diameter of 2 - 4mm.Capillary columns have an internal diameter of a few tenths of a millimeter. They can be one of two types; wall-coated open tubular (WCOT) or support-coated open tubular (SCOT). Wall-coated columns consist of a capillary tube whose walls are coated with liquid stationary phase. In support-coated columns, the inner wall of the capillary is lined with a thin layer of support material such as diatomaceous earth, onto which the stationary phase has been adsorbed |
| Column temperature: For precise work, column temperature must be controlled to within tenths of a degree. The optimum column temperature is dependant upon the boiling point of the sample. As a rule of thumb, a temperature slightly above the average boiling point of the sample results in an elution time of 2 - 30 minutes. Minimal temperatures give good resolution, but increase elution times. If a sample has a wide boiling range, then temperature programming can be useful. The column temperature is increased (either continuously or in steps) as separation proceeds. |
| Detectors: There are many detectors which can be used in gas chromatography. Different detectors will give different types of selectivity. A non-selective detector responds to all compounds except the carrier gas, a selective detector responds to a range of compounds with a common physical or chemical property and a specific detector responds to a single chemical compound. Detectors can also be grouped into concentration dependant detectors and mass flow dependant detectors. The signal from a concentration dependant detector is related to the concentration of solute in the detector, and does not usually destroy the sample Dilution of with make-up gas will lower the detectors response. Mass flow dependant detectors usually destroy the sample, and the signal is related to the rate at which solute molecules enter the detector. The response of a mass flow dependant detector is unaffected by make-up gas. Have a look at this tabular summary of common GC detectors: |
| B. IR Gas analyzer: An infrared gas analyzer measures trace gases by determining the absorption of an emitted infrared light source through a certain air sample. Trace gases found in the Earth's atmosphere get excited under specific wavelengths found in the infrared range. The concept behind the technology can be understood as testing how much of the light is absorbed by the air. Different molecules in the air absorb different frequencies of light. Air with lots of a certain gas wil l absorb more of a certain frequency, allowing the sensor to report a high concentration of the corresponding molecule. |
| IR Gas analyzer can be of two types |
| 1. Non-Dispersive IR-Analyzer |
| 2. Dispersive s IR analyzer |
| Principle |
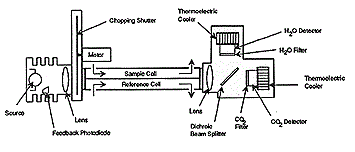
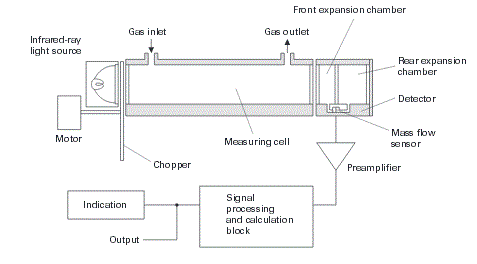
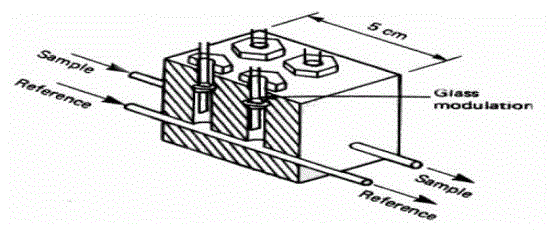
| The main components are an infrared source (lamp), a sample chamber or light tube, a wavelength sample chamber, and gas concentration is measured electro-optically by its absorption of a specific wavelength in the infrared (IR). The IR light is directed through the sample chamber towards the detector. In parallel there is another chamber with an enclosed reference gas, typically nitrogen. The detector has an optical filter in front of it that eliminates all light except the wavelength that the selected gas molecules can absorb. Ideally other gas molecules do not absorb light at this wavelength, and do not affect the amount of light reaching the detector to compensate for interfering components. For instance, CO2 and H2O often initiate cross sensitivity in the infrared spectrum. As many measurements in the IR area are cross sensitive to H2O it is difficult to analyze for instance SO2 and NO2 in low concentrations using the infrared light principle. |
| The IR signal from the source is usually chopped or modulated so that thermal background signals can be offset from the desired signal. A non-dispersive infrared sensor (or NDIR) sensor is a simple spectroscopic device often used as gas detector. It is called non-dispersive because wavelength which passes through the sampling chamber is not pre-filtered instead a filter is used before the detector. |
| C. Thermal conductivity gas analyzer: Each gas has the ability to conduct heat at a specific rate. This is known as the thermal conductivity of the gas. This property is utilized in the thermal Conductivity detector. Heated metal filaments are exposed to the zero and sample gases. The amount of heat carried away by the gases changes the rate of cooling of the wire filament and, therefore its temperature. This temperature change causes a resistance change. Since the filaments are arranged in a Wheatstone bridge, the resistance change can be converted to an electrical current that is available as an output signal. |
| Applications |
| The thermal conductivity analyzer has wide application in industry where it is desirable to analyze a two gas mixture. The thermal conductivity method is very stable in regards to span and zero drift. Applications include welding shield gas mixtures, food packaging mixtures, leak detection mixtures and heat treating atmosphere. Each gas has a known thermal conductivity. Thermal conductivity is measured with a sensor that employs four matched filaments that change resistance according to the thermal conductivity of the gas passing over it. The thermal conductivities of some gases can be found in the table 2 below. |
| Principle of Operation for thermal conductivity analysis |
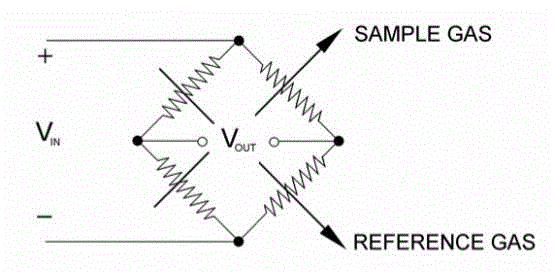
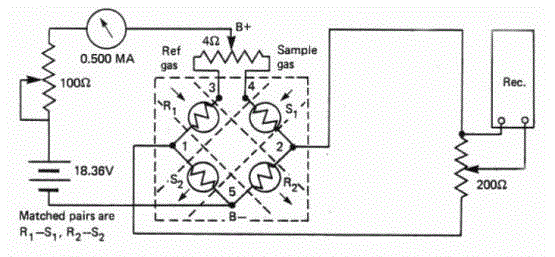
| The gas analyzer sensor uses four matched filaments that change resistance according to the thermal conductivity of the gas passing over it. These four filaments are connected in a Wheatstone Bridge configuration as shown below |
| When all four resistances are the same, VOUT is zero and the bridge is considered balanced. When zeroing, the reference gas is passed over all the filaments, the resistances will be the same (because filaments are matched) and the bridge is balanced. When the sample gas is passed over half of the bridge, then V’s value correlates to the content of the sample gas in the reference. The detector is a four element Katharometer having two elements situated in the reference gas and two elements in the sample gas shown in Figure 5 below. |
| The four elements are electronically connected in a bridge circuit and a constant current is passed through the bridge to heat the elements. If each element is surrounded by the same gas, then the temperature and hence the resistance of each element will be similar and the bridge circuit will be balanced. |
| When the gas to be measured is introduced into the sample gas stream, the two Katharometer elements in this gas stream will be cooled to a greater extent than the two elements in the reference gas. The bridge circuit will be unbalanced, producing a signal voltage related to the measure gas content of the sample gas. This relationship is non-linear. |
| Applications |
| Measure the gas sample content of a sample/reference mixture by comparing the thermal conductivity of the mixture with that of a reference. For example, hydrogen has a thermal conductivity which is approximately seven times greater than that of nitrogen, so small changes are readily detected. All other common gases have thermal conductivities similar to nitrogen so the method of measurement is fairly selective. Helium is the only other gas with a thermal conductivity comparable with that of hydrogen. Other gases that may be measured using this technique are: |
| • Carbon Dioxide |
| • Oxygen |
| • Argon |
| • Methane |
| • Sulphur Dioxide |
| • Ammonia |
| Applications range from high purity gas production to furnace atmospheres. |
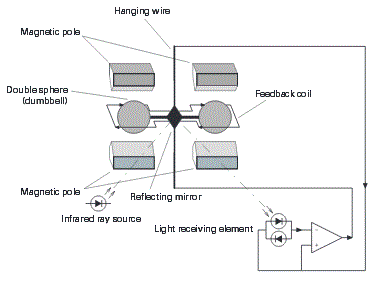
| D. Paramagnetic type gas analyzer: This analyzer is a dumbbell type paramagnetic oxygen analyzer. Because this analyzer is based on that the magnetic susceptibility of oxygen gas is larger than coexisting gases, stable measurement is ensured unaffected by coexisting gases. The detector does not have a heating part such as heater. Therefore, this analyzer is suited for measuring the oxygen concentration in flammable gas. Further, running cost can be saved since reference gas is not required. |
| The principle of measurement is dependent on the strong magnetic property of oxygen molecules. Therefore, measurement is almost unaffected by other molecules weaker in magnetic property than oxygen. Some of its features are as follows: |
| 1. Suited for measuring oxygen in flammable gas. |
| 2. Small-sized and easy to handle. |
| 3. Usable with a wide range of power supplies. |
| 4. Output is linear |
| In the cell, two glass spheres filled with nitrogen gas are suspended with strong metal. At first, the spheres are kept in balance in an in homogeneous magnetic field. When oxygen molecules having a large magnetic susceptibility flow there, the molecules are pulled toward the stronger magnetic field zone and the spheres are moved away from the zone. The resulting deviation of the spheres is detected with the light source, reflecting mirror and light receiving element, and a current is flowed through the feedback loop to control so that the spheres can return to the initial balanced state. The current flowing through the feedback loop is proportional to oxygen concentration. Thus, oxygen concentration is converted into an electric signal |
| E. Electrochemical analyzer: Electrochemical gas sensors are gas detectors that measure the concentration of a target gas by oxidizing or reducing the target gas at an electrode and measuring the resulting current. Beginning his research in 1962, Mr. Naoyoshi Taguchi became the first person in the world to succeed in the development of a semiconductor device which could detect low concentrations of combustible and reducing gases when used with a simple electrical circuit. Devices based on this technology are often called "TGS" (Taguchi Gas Sensors). |
| Construction |
| The sensors contain two or three electrodes, occasionally four, in contact with an electrolyte. The electrodes are typically fabricated by fixing a high surface area precious metal on to the porous hydrophobic membrane. The working electrode contacts both the electrolyte and the ambient air to be monitored usually via a porous membrane. The electrolyte most commonly used is a mineral acid, but organic electrolytes are also used for some sensors. The electrodes and housing are usually in a plastic housing which contains a gas entry hole for the gas and electrical contacts. |
| Operation |
| The gas diffuses into the sensor, through the back of the porous membrane to the working electrode where it is oxidized or reduced. This electrochemical reaction results in an electric current that passes through the external circuit. In addition to measuring, amplifying and performing other signal processing functions, the external circuit maintains the voltage across the sensor between the working and counter electrodes for a two electrode sensor or between the working and reference electrodes for a three electrode cell. At the counter electrode an equal and opposite reaction occurs, such that if the working electrode is an oxidation, then the counter electrode is a reduction. |
| F. ORSAT apparatus |
| An Orsat gas analyzer is a piece of laboratory equipment used to analyze a gas sample (typically fossil fuel flue gas) for its oxygen, carbon monoxide and carbon dioxide content. Although largely replaced by instrumental techniques, the Orsat remains a reliable method of measurement and is relatively simple to use. |
| Construction |
| The apparatus consists essentially of a calibrated water-jacketed gas burette connected by glass capillary tubing to two or three absorption pipettes containing chemical solutions that absorb the gasses it is required to measure. For safety and portability, the apparatus is usually encased in a wooden box. |
| The absorbents are: |
| a) Potassium Hydroxide (Caustic Potash) |
| b) Alkaline pyrogallol |
| c) ammoniacal Cuprous chloride |
| The base of the gas burette is connected to a leveling bottle to enable readings to be taken at constant pressure and to transfer the gas to and from the absorption media. The burette contains slightly acidulated water with a trace of chemical indicator (typically methyl orange) for coloration. |
| Method of analysis |
| By means of a rubber tubing arrangement, the gas to be analyzed is drawn into the burette and flushed through several times. Typically, 100mls is withdrawn for ease of calculation. Using the stopcocks that isolate the absorption burettes, the level of gas in the leveling bottle and the burette is adjusted to the zero point of the burette.The gas is then passed into the caustic potash burette, left to stand for about two minutes and then withdrawn, isolating the remaining gas via the stopcock arrangements. The process is repeated to ensure full absorption. After leveling the liquid in the bottle and burette, the remaining volume of gas in the burette indicates the percentage of carbon dioxide absorbed.The same technique is repeated for oxygen, using the pyrogallol, and carbon monoxide using the ammoniacal cuprous chloride. |
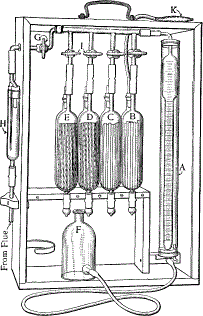
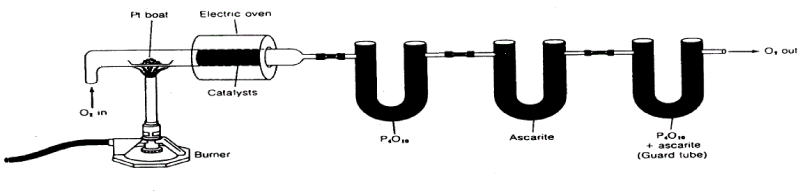
| G. Gravimetry gas analyzer: Total carbon and hydrogen can be determined in a solid by combustion in the presence of pure, dry oxygen (Figure 7.1). The combustion gases are oxidized completely to CO2 and H2O with the aid of a transition metal catalyst. The first trap, phosphorus pentoxide (P2O5), efficiently removes all water formed. Recall that P2O5 is deliquescent, and an efficient desiccant. The second contains ascarite, which is sodium hydroxide-impregnated asbestos. This will trap all of the carbon dioxide. The third tube protects the other two from backflow of atmospheric water and carbon dioxide. After the sample is completely oxidized, the tubes are weighed to determine the increase. This type of analysis is sometimes conducted on dried sludges, sediments, soils, and extracted aquatic organic matter. |
| H. Methanometer: The methanometer is an instrument to measure the percentage of methane in the air in underground Coal mines and has been designed to alert miners to the presence of potentially dangerous concentrations of this gas. The first electrical methanometer for use in coal mines was developed in 1949. It was known as the W8 methanometer and was powered by an Edison cap lamp battery. Several types of hand held electronic methanometers were developed around the world during the 1950's but the first independently powered instrument, the GP (general purpose) methanometer, was not introduced until 1961. |
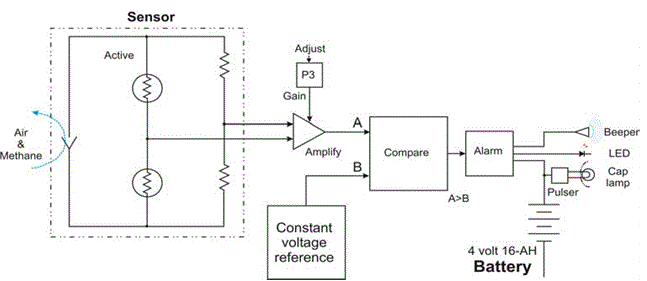
| Methane gases are highly combustible in nature. It burns very quickly when it comes in contact with oxygen. Methane ignitions that have occurred in mine out by areas indicate the need to provide better protection to workers. Handheld methane monitors are now used by some miners to make periodic measurements of methane at the working face. Methanometer is one of instrument used as methane gas detector which is incorporated into a miner’s cap lamp and worn on a miner’s helmet can continuously provide an alarm signal whenever methane levels exceed a set level. Tests were conducted to evaluate the performance characteristics of this methane detector by measuring response times with methane gas supplied through a calibration fixture or adaptor. Other response time tests were performed with the detector in an environmental test box. Performance was also evaluated in a full scale test gallery where face methane emission and underground ventilation |
| The highest methane concentrations in gassy coal mines are most likely to occur near active mining faces. Methane in air mixtures can be ignited if the methane concentrations are between 5 and 15 pct by volume Methane gas can also accumulate in areas outby the face due to slow liberation in poorly ventilated areas, a sudden outburst of gas from the seam, or a migration of gas from worked out areas. |
| No regular monitoring for methane is required in these areas. When working in or traveling through these outby areas, workers can be exposed to ignitable mixtures of methane without any warning. Gas detector is designed to be worn by miners and provide them with an alarm whenever methane levels exceed a predetermined limit. It is designed to alert them with both visual and aural alarms when methane concentrations are excessive. |
| |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
| |
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
 |
 |
 |
 |
| Figure 5 |
Figure 6 |
Figure 7 |
Figure 8 |
 |
 |
 |
| Figure 9 |
Figure 10 |
Figure 11 |
|
| |
References
|
- Kevin Harris , Randy Hauer , Dan Potter “Combustibles measurement in sulfur recovery unit acid gas with a combined nduv/ndiranalyzer” Analysis Division Symposium at ISA AD 2008; http://www.isa.org
- Matese. A, Gioli, B., Vaccari F. P., Zaldei A., and Miglietta, F. (2009) Carbon Dioxide Emissions of the City Center of Firenze, Italy: Measurement, Evaluation, and Source Partitioning. Journal of Applied Meteorology and Climatology (48), 1940-1948.
- Ocheltree, T. and Loescher H “General procedures for CO2 and H2O calibration of Infrared Gas Analyzers (IRGA) and Secondary Gas Standards “ (2010) Online
- Joseph E. Chilton, Charles D. Taylor, and Robert J. Timko “EVALUATION OF IYONI II METHANOMETERS” National Institute for Occupational Safety and Health/Pittsburgh Research Lab Pittsburgh, PA USA
- Fillmer W. Ruegg and Carl Halpern “Gravimetric Analysis of Exhaust Gas From Gas Turbine Combustion Chambers” Journal of Research of the National Bureau of Standards Volume 45,No.2, August 1950
|