ISSN: 2320-2459
ISSN: 2320-2459
A Haddad*, A Hafidi, N Chahmat, A Ain-Souya, R Ganfoudi, and M Ghers
Faculty of Sciences, Department of Physics, LESIMS, PB12 University, Badji Mokhtar –Annaba, Algeria.
Received date: 11/06/2013 Revised date: 17/06/2013 Accepted date: 23/06/2013
Visit for more related articles at Research & Reviews: Journal of Pure and Applied Physics
The regeneration of binary semiconductor layers after isothermal adsorptions of oxygen at various temperatures carried out between 20°C and 350 °C has been studied. The used samples are layers of CdSe obtained by vacuum evaporation on glass substrates, ZnO and SnO2 oxide layers. These last were grown by oxidation of Zn and Sn layers at respective temperatures of 450°C and 200°C under O2 gas. The considered layers of metals were prepared by the techniques of vacuum evaporation on glass, alumina and metal substrates, and electrodeposition on metal substrates of various natures (copper, aluminium, steel…). The experimental results show that during adsorption of oxygen, the electric resistance measured between two points of the samples surface varies as a function of the temperature and the nature of the samples. The layers of CdSe and ZnO strongly adsorb oxygen at high temperatures around 200°C, while the rate of maximum adsorption of O2 by SnO2 is obtained at lower temperatures. The isothermal desorption carried out at the same temperatures of adsorption show that the layers can be regenerated but for relatively long lengths of time. The layers reheated under O2, at temperatures chosen, are less sensitive to this element. Total regeneration proves the reversible nature of the oxygen interaction with surface and informs about the stability of the material
adsorption, conductance, desorption, oxygenate, semiconductor surfaces.
The layers of CdSe and ZnO strongly adsorb oxygen at high temperatures around 200°C, while the rate of maximum adsorption of O2 by SnO2 is obtained at lower temperatures. The isothermal desorption carried out at the same temperatures of adsorption show that the layers can be regenerated but for relatively long lengths of time. In the event of insufficiency, the samples are subjected to a desorption programmed in temperature by sweeping of the temperature at relatively slow speed of 3°C/mn, which allows a differential contribution of energies of desorption. Those are highlighted by the curves S (T) = d Log R / d (103/T) . The layers reheated under O2, at chosen temperatures, are less sensitive to this element. Total regeneration shows the reversible nature of the reaction of interaction of oxygen with surface and informs about the stability of material. With an aim of producing gas sensors in the medium term, it appeared interesting to use as adsorbing material a thin layer, whose specific surface, considerably more important than that of a monocrystal of the same apparent surface, should confer on the conduction of the surface layer a better sensitivity to strong chemisorption of gases [1,2,3,4,5].
In the simple case, one can say that the gas while being fixed on a semiconductor surface introduces into the forbidden band of the material a surface state which can play the part of acceptor or donor of electrons: if it is acceptor the surface takes a negative electric charge, while if it is donor it takes a positive electric charge. This surface charge must be compensated under the semiconductor surface by a zone of space charge of opposite sign whose nature (accumulation, impoverishment), depends on type n or p of the semiconductor. The space charge modifies the conductance of the surface zone of the solid and consequently the total resistance of the semiconductor plate. Note that the relative variation of resistance for a single-crystal layer of a semiconductor of the type n is given by:
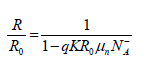
Where R0 is initial electric resistance, q the electronic charge, μn the mobility of the electrons, varies and K a dimensional constant of the layer 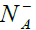 varies according to time during isothermal adsorptions and desorptions until electronic equilibrium is reached in both cases. We will use then, the resistance variation with the temperature and time as a parameter of gas interaction.
varies according to time during isothermal adsorptions and desorptions until electronic equilibrium is reached in both cases. We will use then, the resistance variation with the temperature and time as a parameter of gas interaction.
The curves in Figs (1), (2) and (3) for the isotherm adsorption / desorption (namely, at the same temperatures) show that, generally, desorption is not complete. There is some temperature values favors desorption. Energy made by them correspond to the energy required for desorption at maximum density of ionosorbed species. But a high density of the adsorbate can be desorbed by these energies.
The curves in Figs (4) and (5) show that the sensitivity of the samples to oxygen is favored in certain temperature ranges. The layers of ZnO strongly adsorb oxygen at high temperatures around 200 ° C, while the maximum rate of adsorption of O2 on SnO2 is obtained at lower temperatures.
In this work, we have found that the layers prepared under different conditions possess a behavior of a n-type semiconductor. We also showed that the resistance at room temperature, is high on the order of (M Ω) for ZnO and SnO2 deposited on glass substrates and (GΩ) for CdSe, and varies significantly with temperature. These samples are sensitive to the presence of oxygen in the temperature range from ambient to 350°C. Tests show that adsorption of oxygen, in the case of different types of samples, ionosorption of this gas causes a large change in electrical resistance temperature dependent. Additionally, we found that the temperatures corresponding to maximum variations, as those relating to high reactivity of surface with oxygen, these states are activated to the energy required for interaction with this type of gas.