Jefry Winner1*, Chevanthi Priya2
1Department of Pharmacology, The Jawaharlal Institute of Postgraduate Medical Education & Research (JIPMER), Puducherry, India
2Department of Neurology, Vinayaka Missions Research Foundation (VMRF), Salem, Tamil Nadu
Date of Submission: 01 Aug, 2022, Manuscript No. jnhs-22-68474; Editor Assigned: 03 Aug, 2022, Pre QC No. P-68474; Reviewed: 17 Aug, 2022, QC No. Q-68474; Revised: 24 Aug, 2022, Manuscript No. R-68474; Published: 31 Aug, 2022, DOI: 10.4172/JNHS.2022.8.8.38
Visit for more related articles at Research & Reviews: Journal of Nursing and Health Sciences.
COVID-19 pandemic has become self-explanatory among with its hallmark havoc that it caused to people all over the world. The damage it caused is almost surpassing the world wars combined and became a global pandemic in all aspects of life. COVID-19 has become a word of threat in almost many parts of the world. Like many other viral illnesses this was treated conservatively, but due to lack of man power and the war like situation during COVID-19 there was no place for good research as it is a time-consuming process. This led to physicians and policymakers rely on existing and previous knowledge to treat the infection. Numerous drugs were tried and some of them were proven to be beneficial rest faded away in time. In this review we have tried to analyse the various drugs used in COVID-19 and the adverse effects that could be potentially attributable to them. To identify and inform regarding them to their superiors is the shared responsibility of nurses with physicians in this pandemic situation. It is essential as every healthcare professional is overburdened. This could help in the betterment and safety to be sustained in healthcare and improved reporting and timely management of ADR.
ADR, Nursing, Pharmacology, Clinical pharmacology
Nurses are of immense importance to healthcare system in any part of the world. There role is inevitable in many aspects of healthcare. They are mainly involved as the ones who work along with the team physicians and help in accomplishing the goal they are up to. In the midst of COVID-19 there is a heavy shortage of manpower in healthcare system in many parts of the world [1]. This has been a major blow in managing patients with COVID-19. This not only has called for sharing responsibilities among healthcare workers. One of the major areas often neglected in health care setup is regarding the ADRs that occur following administration of drugs. In COVID-19 due to lack of effective pharmacotherapy many drugs have been tried without great evidence. One among them was remdesivir and then some others were monoclonal antibodies [2]. With these factors in mind, it is important to have a watch for adverse drug reactions in patients treated with those drugs. Another drug used extensively in COVID-19 were the corticosteroids [3]. They were found to be mortality reducing drugs which further augmented their use [4]. These corticosteroids were known to cause numerous side effects. So, monitoring of patients is important and this could be done well by nurses who are involved in patient care. Knowledge regarding the common and rare adverse effects is essential for the purpose of identifying them in these patients. And the short term as well as long term adverse effects should be known by them to suspect and watch out for. This service rendered by nurses is a part of shared responsibility with doctors and this approach could make a change by early and effective reporting of ADRs in health care setup and appropriate management of patients.
United States of America
One of the countries to have so much suffered from COVID-19 is USA. Despite all advancements they had it was a nightmare for them when it came to handling of COVID-19 infection. After the first case being reported in Wuhan China in December 2019 USA has seen a drastic rise in cases, until today as per the WHO report there were 81.6 million confirmed cases of COVID-19 as of May 2022 [5].
United Kingdom
This is another important nation to be affected by COVID-19. The management of COVID-19 illness was a quite challenging task for the authorities and health care workers. In UK from the first case reported to till this day there were 22 million cases reported as per WHO and 177 thousand deaths were recorded until May 2022 [6].
Europe
The pandemic of COVID-19 also had its impact in the European soil or rather in the air to say it aptly. Even before the pandemic was declared by the World Health Organization (WHO), the cases of COVID-19 were already beginning to significantly increase in some European states and then ended up involving the continent as a whole. As of January 2021, 84,532,824 confirmed cases were recorded in the world; in the European Union and the European Economic Area (EU/EEA) 15,857,298 cases and 1,845,597 deaths were recorded [7]. As of May 2022, the nation’s namely Germany and France had recorded the highest number of cases and deaths followed by Italy [8].
Asia
This is the place where COVID-19 was believed to be originated from, The Association of South-East Asian nations (ASEAN) countries that include Brunei, Cambodia, Indonesia, Laos, Malaysia, Myanmar, Philippines, Singapore, Thailand, and Vietnam had received severe blow due to COVID-19. Cambodia, Laos, Thailand and Vietnam were all observed to report similar trends with large increases of transmission beyond April 2021. Brunei was observed to report increasing trends beyond July 2021. Singapore and Myanmar were observed to have two distinct waves of transmission of COVID-19 while, Malaysia, Indonesia and the Philippines had three distinct waves of transmission. Trends of daily per capita cases were observed to peak between July and September 2021 for all countries within the ASEAN region. The highest daily per capita cases of 67 cases per 100,000 population was observed in Malaysia [9]. As of May 2022, there were less cases reported in all these nations, but still there are active cases in there [10].
India
In India again the first wave of COVID-19 was little bit within control. But the next wave hit the nation very badly and the healthcare system was stunned. The deaths were expected to be very high compared to the second wave [11]. The nation was affected so badly and is still recovering. As of May 2022, there were around 2000 cases which was very low as compared to the second wave 1 year back were 4 lac cases were reported [12]. The unexpected rise and fall in the COVID-19 cases in India have kept the health authorities vigilant to prevent further surge of COVID-19 cases that might make the healthcare system of the country fumble.
With the lack of concrete evidence and emergency situation there were many drugs used in a trial-and-error basis. There were lot of variations between treatment guidelines followed by different nations.
NIH guidelines
The treatment of COVID-19 is quite concordant with our former statement. As per the NIH COVID-19 treatment guidelines in case of non-hospitalized patients who are stable and does not require oxygen the pharmacotherapy is as follows;
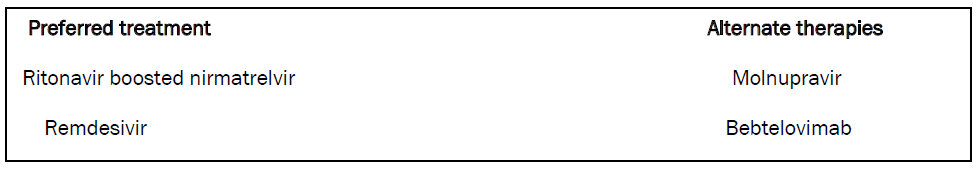
In case of patients who require oxygen there is conflicting evidence and the drugs recommended are as follows [13];
• Dexamethasone
• Remdesivir
In case of hospitalized patients, the NIH guidelines are quite different and the use of steroids and remdesivir and steroids are not recommended. In these cases, only general or conservative management is done
In case of hospitalized patients who require supplemental oxygen and in cases of ECMO the pharmacotherapy includes [13]
• Dexamethasone
• Remdesivir and
• Tocilizumab or Baricitinib
If baricitinib and IV tocilizumab are not available or not feasible to use, tofacitinib can be used instead of baricitinib and IV sarilumab can be used instead of IV tocilizumab.
In case of MIS-C in children the treatment included pharmacotherapy with mainly [13]
• Glucocorticoids
• IVIG
• Anakinra
• Infliximab and
• Aspirin or enoxaparin
As per the NIH guidelines we found out these were the drugs recommended for use as pharmacotherapy in management of COVID-19 illness.
IDSA guidelines
In case of this guidelines from Infectious Disease Society of America they recommend the use of dexamethasone in case of severe COVID-19 with spo2 <94.
The use of tocilizumab is also recommended by the panel and if tocilizumab is not available sarilumab is recommended in case progressive severe or critical illness [14].
Severe illness is defined as patients with SpO2 ≤ 94% on room air, including patients on supplemental oxygen.
Critical illness is defined as patients on mechanical ventilation and ECMO. Critical illness includes end organ dysfunction as is seen in sepsis/septic shock. In COVID-19, the most commonly reported form of end organ dysfunction is ARDS.
They also recommend the use of convalescent plasma in early phase of the disease. They also suggest the use of Remdesivir in patient who have high risk of disease progression. Remdesivir is also recommended in patients who are on supplemental O2 but not on ECMO [14]. IDSA suggests that use of pre-exposure prophylaxis with antibodies tixagevimab/cilgavimab is beneficial. And post exposure prophylaxis with casirivimab/imdevimab is also recommended. bamlanivimab/etesevimab and sotrovimab are recommended in patients who have risk of progression to severe disease. In patients with increased inflammatory markers baricitinib is indicated. Again, Tofacitinib is indicated in patients with severe COVID-19 not on invasive or non-invasive mechanical ventilation [14].
In high-risk patients who are expected to have disease progression Nirmatrelvir/Ritonavir is recommended within 5 days of symptom onset. Molnupravir is again recommended in patients with high risk of disease progression.
European centre for disease prevention and control
Systemic corticosteroids are indicated only in severe cases of COVID-19. Inhalational budesonide is found to be beneficial in reducing hospitalization [15,16]. They recommended the use of tocilizumab and sarilumab in adult patients on mechanical ventilation as well as on corticosteroids [17]. EMA also recommends the use of Casirivimab and imdevimab in case of patients who have high risk of progressive disease who are > 10 years of age. Sotrovimab and regdanvimab was also used in patients similar to the former ones [18].
Molnupravir EMA advised that it can be used to treat adults with COVID-19 who do not require supplemental oxygen and who are at increased risk of developing severe COVID-19. It should be administered within five days of symptom onset [19]. Nirmatrelvir/ Ritonavir was approved as EUA (Emergency Use authorization) by EMA (European Medical Agency).
WHO guidelines
The latest guidelines with revision were released by WHO in April 2022. In these guidelines some recent changes were incorporated. In WHO guidelines the drugs are divided and allocated to different groups of patients depending upon the severity [20].
These are the Pharmacotherapeutic agents that are recommended by WHO latest guidelines. Some of the drugs are recommended as not to be used in COVID-19 those are not mentioned here as it is beyond the scope of the article.
Indian guidelines
In India the COVID-19 treatment guidelines were put forward by MOHFW (Ministry of Health and Family Welfare) and AIIMS (All India Institute of Medical Sciences) [21].
For details regarding the treatment guidelines refer [22]. These are the drugs that are used as per the Indian guidelines and the most common among them are again corticosteroids.
The main purp3nsible for obesity [23] (Figure 1).
ADR monitoring during COVID-19 in patients on corticosteroids: In case of patients on short-term steroid therapy they should be monitored with:
• Blood glucose levels
• Blood pressure
• Waist circumference or weight measurement
• Epigastric discomfort
In case of long-term therapy or in post COVID-19 with corticosteroids patients should be monitored with:
• Complete blood counts
• Ocular examination
• History of seizures
• Any bony dysplastic disorders
These are the effects of steroids every healthcare worker should be aware of and has to be able to suspect and identify them. Knowing these adverse effects by nurses apart from physicians would help in effective identification treatment and reporting of ADR.
Antivirals In Covid-19
There are some important antivirals used in case of COVID-19. Usually, viral illnesses are treated by conservative or supportive management. The reason behind limited use of antivirals is attributed to their side effects and rapid emergence of resistance. But still some of the antivirals have been used in COVID-19 [24].
Remdesivir
This antiviral drug has got EUA by FDA following conflicting evidence in few studies. But it became one of the commonly used drugs in COVID-19. The common adverse effects include
• Weakness
• Gastritis
• Hepatitis
• Cumulative toxicity due to renal failure (due to excipient)
Idiosyncratic and long-term adverse effects include
• Bradycardia [25]
• Hypersensitivity
• Infusion related reactions
Pharmacology of Remdesivir: Remdesivir is a nucleotide analogue that acts by inhibition of RNA dependent RNA polymerase. Its active metabolite is a nucleotide triphosphate, this metabolite can interfere with SA nodal tissue and a suggested mechanism for bradycardia. The metabolism also occurs in the liver by cytochromes and any polymorphisms associated with it may affect the metabolism and the CYP2C8 (minor), CYP2D6 (minor), CYP3A4 (minor), OATP1B1/1B3 (SLCO1B1/1B3), P-glycoprotein/ABCB1 (minor) [26]. So potential drug inducers have to be avoided in these patients. The excipient Sulfobutylether-beta-cyclodextrin sodium salt (SBECD) is present in Remdesivir and has to be cleared renally [27]. This accounts for cumulative toxicity in patients with renal impairment.
Adverse effects monitoring during COVID-19:
These include:
• Slow infusion to avoid sudden hypersensitivity reactions
• Assess for liver function
Adverse effects monitoring post-COVID-19:
These include:
• Bradycardia which requires EEG monitoring
• Liver failure if taken for periods more than prescribed
• Seizures are also reported in some patients
Nirmatrelvir and ritonavir
This is another antiviral drug used in COVID-19 especially in western world. WHO also recommended this for use in COVID-19. This was approved by FDA from the results of EPIC-HR trial (NCT04960202) and A trial called PANORAMIC trial (EudraCT Number: 2021-005748-31) conducted in UK tested its efficacy in COVID-19 and was also approved in UK after that [28].
Pharmacology of Nirmatrelvir and ritonavir: This acts by protease inhibition activity [29]. Ritonavir is again a protease inhibitor whose use is mainly limited to enzyme inhibiting activity against CYP3A4 and used for boosting of other antivirals [30]. This is metabolized by CYP3A4.
The adverse effects of nirmatrelvir and ritonavir includes;
• Dysgeusia
• Myalgia
• Hypertension
• Diarrhoea
Since this is a new drug and most of the information regarding adverse effects will be available only after many clinical trials and post-marketing surveillance the adverse effects listed in this review are limited and might change in the future. The monitoring of these adverse effects during COVID is necessary as like for other drugs.
Molnupravir
This was another new drug that has acquired EUA for COVID-19. This was again approved based on clinical trials especially the “MOVe-OUT trial” (NCT04575597) [31].
Pharmacology of Molnupravir: This antiviral agent is a prodrug of ribonucleoside analogue β-D-N4-Hydroxycytidine 5′-triphosphate. This acts by inhibition of RNA dependent RNA polymerase [32].
Adverse effects: From the clinical trials that have been held for this drug the side effects reported were:
• Diarrhoea
• Nausea
• dizziness
Again, limited data is available since this is been recently approved and post marketing data is unavailable.
Immunomodulators: These are another group of drugs that are commonly used from our previous knowledge gained from various treatment guidelines. They are mostly used in cases of severe to critical cases of COVID-19 who were suspected to develop Systemic Inflammatory Syndrome.
Tocilizumab
Pharmacology: This was a monoclonal antibody directed against IL-6. This binds to soluble as well as bound IL-6 receptors rendering them inactive [33]. This is another drug that was given EUA followed by the former ones.
Adverse effects:
• lURTI
• Transaminitis
• Skin rash
• Anaphylaxis [34]
Long term adverse effects include:
• Gastritis
• Mucosal ulceration
• GI perforation
So, the adverse events monitoring during COVID includes:
• Liver function testing
• Slow infusion followed by test dose
• Avoiding concomitant use with steroids
The ADRs to be monitored post-COVID includes:
• Epigastric discomfort or pain
• Any bleeding per rectum
• Ulcers in oral mucosa
Sarilumab
This is another drug used in places as a substitute to tocilizumab. This has similar mechanism of action like tocilizumab and was approved following the “REMAP-CAP trial” (NCT02735707) [35].
Adverse effects from trial included:
• Neutropenia
• Thrombocytopenia
• URTI and UTI
• Hyperlipidaemia
• Herpes reactivation
Monitoring for ADR includes:
• Complete blood counts
• Lipid profile
• Avoided in patients with previous herpes infection. If necessary, used with caution with periodic oral assessment for lesions.
Sotrovimab
This is another drug that is used for COVID in some places of the world. Sotrovimab has demonstrated activity via two antiviral mechanisms in vitro, antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) [36]. The full analysis of the COMET-ICE (NCT04545060). Trial showed this is better than placebo against omicron variant [37]. The adverse effects and monitoring are same as the previous one. Drug specific ADRs are yet to be known since this is a new drug.
Casirivimab/imdevimab
This is the famous antibody cocktail used in western world. Its use is limited in developing countries because of the cost factor. This was approved in India by CDSCO and FDA in USA. This was evaluated as a part of recovery trial for its effectiveness [38].
Pharmacology: Casirivimab (IgG1-kappa) and imdevimab (IgG1-lambda) are recombinant human monoclonal antibodies (mAbs), which are unmodified in the Fc regions. The mAbs bind to nonoverlapping epitopes of the spike protein receptor binding domain (RBD) of SARS-CoV-2, and thereby block binding to the human ACE2 receptor [39]. This was used in cases of early COVID-19 infection who had high risk of progression to severe disease.
ADRs to watch out for includes:
• Injection site reactions
• Infusion related reactions
• Pneumonia
• Hyperglycaemia
• Nausea
• Vomiting
• Intestinal obstruction
• Dyspnoea
• Anaphylactic reactions
The above reactions are not specific and applies to almost all mAbs and monitoring includes:
• Slow infusion
• Blood glucose monitoring
• Test dose before starting infusion
• Any altered bowel habits
• Abdominal pain
Other drugs used in COVID-19
There are many other drugs used in COVID-19 and these were not discussed as they are less commonly used and only in specified locations the most commonly used drugs and that are used still and recommended by all guidelines are discussed in detail. Some other drugs like azithromycin, hydroxychloroquine was also used initially in some countries and later on evidence proved that they are ineffective or not better than placebo.
This review is aimed at making the healthcare workers and nurses especially in imparting them knowledge regarding the drugs and important adverse effects associated with them. The pandemic has created a situation which could not be handled only by doctors and requires the cooperation of nurses and other healthcare workers. This limitation of this review includes that all drugs or their molecular mechanisms were not explained in detail since this is mostly aimed at early identification and prevention or appropriate treatment of the same. We hope this review would serve as a manual for healthcare workers and aid in ADR reporting with physicians.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at