Medical images are noisy images and its processing is a complex one. The boundary detection of aortas in cardiovascular Magnetic Resonance (MR) images is difficult. This paper presents a vector image and edge map based boundary detection of aortas in cardiovascular MR images and is implemented using MATLAB. The performance of the edge vector and edge map boundary detection method for detecting the boundary of aortas in cardiovascular MR images is compared with an Active Contour Model (ACM). Results shows that the edge vector and edge map based boundary detection model is better than the active contour based model.
Keywords |
| Aortas, boundary detection, edge map, edge vector, image segmentation |
INTRODUCTION |
| Images are used in various applications including medical diagnostics, weather forecasting through satellite images,
surveillance, and non-destructive testing. Image processing is a signal processing unit in which the input is an image
such as photograph and the output may be either an image or set of parameters related to the image [11]. Edge detection
and boundary detection plays a fundamental role in image analysis and computer vision [13]. Boundary map obtained
from boundary detection of an image can give useful information. This information is further useful in image analysis
and interpretation tasks. Boundaries provide a clear outline or shape of an object. Obtaining the correct boundary in an
image is a critical task. Boundary detection needs several steps in computer vision tasks |
| A boundary is a contour in the image plane that represents a change in pixel from one object to another. An edge
may be defined as a significant change in features of image such as color or brightness. Medical image edge detection
is useful for object recognition and needs an essential pre-processing step in image segmentation. Segmentation means
grouping of a set of pixels that share similar characteristics, such as intensity and texture. This refers to the set of pixels
which are mapped from the structures inside the prostate and the background image. |
| Image segmentation is classified into two types, namely, edge based approach and region based approach. Edge
based approach is used with edge detection operator such as Sobel, Laplacian, and Canny for detecting the object
boundary and then extract the boundaries based on the edge information. This approach is center around the contour
detection. Region based approach will partition the image into connected regions and the neighbors are grouped
together based on image properties. Image properties consist of intensity values of the original images or values that are
computationally based on regional image data. The techniques used in this approach use clustering, region growing,
region splitting and region merging. |
| The rest of the paper is organized as follows. Section 2 reviews about the related literature on boundary detection in
medical images and section 3 describes about active contour model used in the boundary detection. Section 4 depicts
the vector image and edge map based boundary detection model for detecting the boundary in medical images. Section
5 details the experimental setup and analysis the simulation results in which the boundary of aortas in cardiovascular
MR images is compared with ACM model. Finally conclusion is given in section 6. |
RELATED WORK |
| In this section, we review the prior work on the various boundary detection models implemented in medical images.
Julian et al [8] presented a method for vessel segmentation and tracking in ultrasound images using Kalman filters.
Modified Star-Kalman technique is used to determine vessel contours and ellipse parameters using an extended Kalman
filter with an elliptical model. A temporal Kalman filter is used for tracking the vessel center over several frames, using
location measurements from a handheld sensorized ultrasound probe. Segmentation and tracking is implemented in
real-time and validated using simulated ultrasound data with known features and real data. Results indicate that the
mean errors between segmented contours and expert tracings are 1 to 2 percent of the maximum feature dimension. |
| Francois et al [3] presented segmentation of intimamedia thickness of carotid arteries in view of computing various
dynamical properties of that tissue, such as elasticity distribution. The echogenicity of a region of interest comprising
the intima-media layers, the lumen, and the adventitia in an ultrasonic B-mode image is modelled by a mixture of three
Nakagami distributions. The method is well suited for semi-automatic context that requires minimal manual
initialization. |
| Juin et al [7] proposed segmentation for brain MR images using a distributed transformation. This method extends
the Gaussian mixtures expectation-maximization segmentation to a power transformed version of mixed intensity
distribution. Different correcting bias is applied before image segmentation to avoid performance degradation caused
by intensity inhomogeneity. The partitions of brain tissues were validated and evaluated against manual segmentation
from the internet brain segmentation repository and simulated image volumes from BrainWeb. |
| Jiantao et al [6] developed an automated method using computational geometry to detect and segment fissures
depicted on Computed Tomography (CT) images. After geometric modelling of lung using marching cube algorithm,
Laplacian smoothing is applied to enhance pulmonary fissures by depressing nonfissure structures while smoothing the
surfaces of lung fissures. An extended Gaussian image method is used to locate the fissures in a statistical manner. The
performance was evaluated by thoracic radiologists using a set of 100 images randomly selected from 10 screening CT
examinations. |
| Veeralakshmi et al [13] proposed an edge detection technique for detecting the correct boundary of objects in an
image. The method can detect the boundaries of object using the information from intensity gradient using the vector
image model and texture gradient using the edge map model. Padmapriya et al [11] proposed a method to detect
boundary in medical image using magnitude, direction, edge map and density of the edges bounded to the object and to
crop the detected object and enlarge the image. The blur effect of the enlarged image is removed and a high resolution
image is produced from the low resolution image. |
| Santhosh et al [12] proposed an external force for active contours called Vector Field Convolution (VFC), to
address problems related to limited capture range, noise sensitivity, and poor convergence to concavities. VFC is
obtained by convolving the edge map generated from the image with the user defined vector field kernel. Mixed VFC is
used to alleviate the leakage problem caused by choosing inappropriate parameters. |
| Jagadish et al [5] proposed an algorithm for medical image segmentation based on vigorous smoothening by
identifying the type of noise and edge diction ideology which seems to be a boom in medical image diagnosis. The
algorithm gets the medical image and pre-processes to remove the noise by suitable filter after identifying the type of
noise and carried out edge detection for image segmentation. The algorithm is very effective with low quality or
marginal vague images which have high spatial redundancy, low contrast and biggish noise. |
| Dinesh et al [1] projected the important place of segmentation of images in extracting information for decision
making. The study reviewed the research on various research methodologies applied for image segmentation and aims
to provide a simple guide to the researcher for those carried out their research study in the image segmentation. |
ACTIVE CONTOUR MODEL |
| Active Contour Model (ACM) is a technique to remove the interferences and artifacts inherent in the medical images.
ACM is also called snakes, which has the ability to efficiently combine both the available knowledge about the
structure of interest and local correspondences with the image features [9]. ACM is developed with a segmentation
strategy that it combines a marker controlled active contour based model and the mathematical morphological
methodology [4]. |
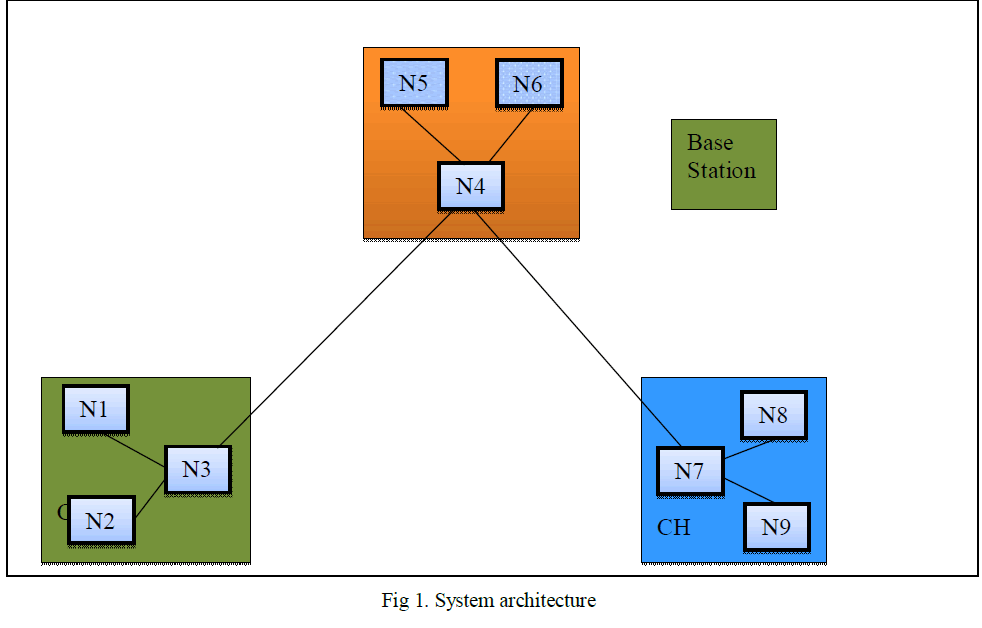
| Figure 1 shows the structure of ACM. An active contour V is defined by an ordered set of n different nodes, V = [v1,
v2, . . . , vn], giving coordinates of points on the contour in a circular manner [9]. The n control points are involved by
using a cubical B-spline curve. For an input image y, the energy function is given by, |
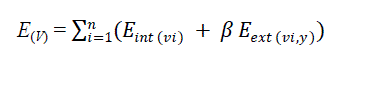 |
| In (1) β is a weighting parameter, Eint is the internal energy term, Eext is the external energy term and vi is the
contour element. The internal energy allows to express the available priori knowledge about the contour shape to be
detected. The external energy allows to pull the ACM towards the desired image features. |
| Detecting correct boundaries in medical images is a difficult task. ACM gives ill-defined edges in medical image,
which is the limitation. This technique also requires proper initialization of the initial contour, which is not too far from
the expected boundary. ACM is also difficult to implement in real-time and often require careful tuning of parameters
for convergence. |
VECTOR IMAGE AND EDGE MAP BASED BOUNDARY DETECTION MODEL |
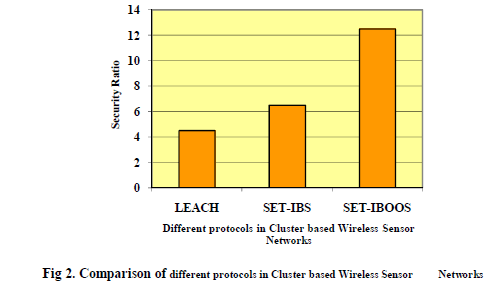
| Figure 2 shows the structure of the vector image and edge map based boundary detection model. Once the input image
is received it is given to average edge vector field model [2] through an initial position section. The initial position is
used to determine a good initial position of edge in the contour model. For an input image f(x, y), the average edge
vector field is computed as, |
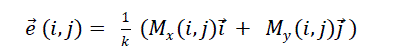 |
| where, |
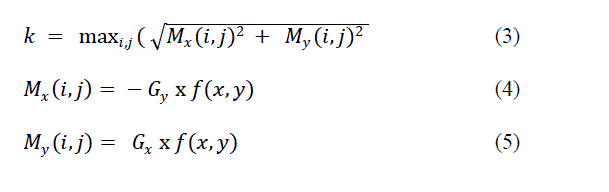 |
| and Gx and Gy are the difference masks of the Gaussian weighted image moment vector operator in the x and y
directions |
| Edge map is obtained as the edges of objects in the medical image. This can be derived from Law’s texture or
Canny edge detection. Law’s texture is computed by convolving an input image with a mask. Canny edge detection is
used for step edges corrupted by white Gaussian noise. This will first convolve the output image obtained from Law’s
texture with a Gaussian filter. Then calculate the magnitude and direction of the gradient. Then nonmaximal
suppression is made to identify edges and broad ridges in the magnitude must be thinned. Finally thresholding is made
to detect and link edges. |
| The boundary of the object is obtained by edge following technique, for this edge magnitude information is
considered. But this is not sufficient in noisy images. The average edge vector field and edge map technique will give
more information for searching the boundary of objects, with increased probability of correctness. |
RESULTS |
| Analysis of an edge vector and edge map based boundary detection approach in medical image has been carried on an
Intel Core 2 Duo CPU system with 2.10 GHz on a 32-bit Windows 7 Ultimate Operating System using MATLAB. The
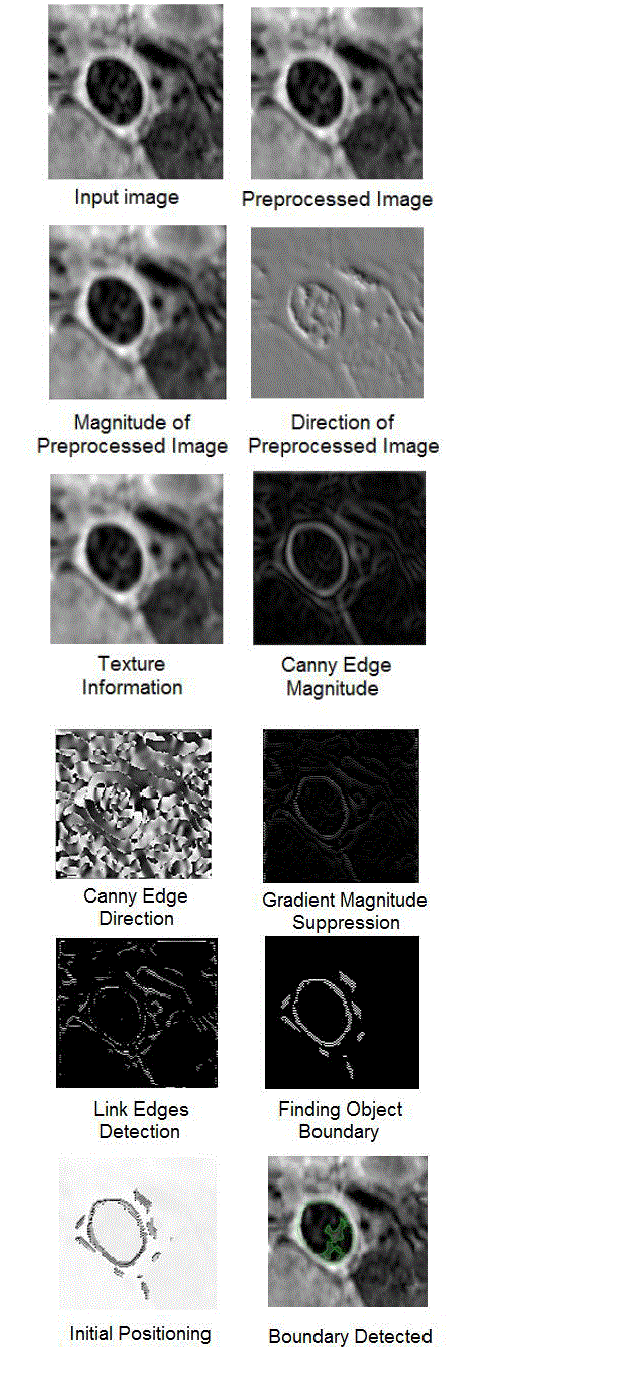
simulation results of each process in vector image and edge map based boundary detection of aortas in cardiovascular
MR images is shown in figure 3. |
| The performance of the vector image and edge map based boundary detection approach is compared with the ACM
approach and obtained the probability of error and computation time. The comparison is shown in table 1. The
probability of error (PE) in image segmentation [14] is obtained by the formula mentioned in (6). |
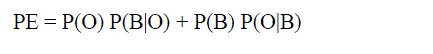 |
| where, |
| P(O) = priori probabilities of objects in the image |
| P(B) = priori probabilities of background in the image |
| P(B|O) = probability of error in classifying objects as background |
CONCLUSION |
| In this paper, a vector image and edge map based boundary detection model for detecting the boundary in a noisy image
is implemented using MATLAB. The performance of the edge vector and edge map boundary detection method for
detecting the boundary of aortas in cardiovascular MR images is compared with an Active Contour Model (ACM).
Analysis has been made for the probability of errors in image segmentation and computation cost. Results shows that
the edge vector and edge map based boundary detection model is better than the active contour based model in
detecting the object boundaries in noisy images. This model can be also used in other applications where ill-defined
boundary detection is required. |
Tables at a glance |
 |
| Table 1 |
|
Figures at a glance |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
|
References |
- Dinesh D. Patil, and Sonal G. Deore, âÃâ¬ÃÅMedical Image Segmentation: A ReviewâÃâ¬ÃÂ, International Journal of Computer Science and Mobile ComputingâÃâ¬ÃÂ, Vol. 2, Issue 1, pp. 22-27, 1st Jan. 2013.
- N. Eua Anant, and L. Udpa, âÃâ¬ÃÅA Novel Boundary Extraction Algorithm based on a Vector Image ModelâÃâ¬ÃÂ, Proceedings of the IEEE Symposium on Circuits and Systems, pp. 597-600, 1996.
- Francois Destrempes, Jean Meunier, Marie-France Giroux, Gilles Soulez, and Guy Cloutier, âÃâ¬ÃÅSegmentation in Ultrasonic B-Mode Images of Healthy Carotid Arteries Using Mixtures of Nakagami Distributions and Stochastic OptimizationâÃâ¬ÃÂ, IEEE Transactions on Medical Imaging, Vol. 28, No. 2, pp. 215-229, February 2009.
- A. Guocheng, C. Jianjun, and W. Zhenyang, âÃâ¬ÃÅA Fast External Force Model for Snake-based Image SegmentationâÃâ¬ÃÂ, Proceedings of the International Conference on Signal Processing, pp. 1128-1131, 2008.
- Jagadish H. Pujar, Pallavi S. Gurjal, Shambhavi D. S., and Kiran S. Kunnur, âÃâ¬ÃÅMedical Image Segmentation based on Vigorous Smoothing and Edge Detection IdeologyâÃâ¬ÃÂ, International Journal of Electrical and Computing Engineering, Vol. 5, Issue 2, pp. 121-127, 2010.
- Jiantao Pu, Joseph K. Leader, Bin Zheng, Friedrich Knollmann, Carl Fuhrman, Frank C. Sciurba, and David Gur, âÃâ¬ÃÅA Computational Geometry Approach to Automated Pulmonary Fissure Segmentation in CT ExaminationsâÃâ¬ÃÂ, IEEE Transactions on Medical Imaging, Vol. 28, No. 5, pp. 710-719, May 2009.
- Juin-Der Lee, Hong-Ren Su, Philip E. Cheng, Michelle Liou, John A. D. Aston, Arthur C. Tsai and Cheng-Yu Chen, âÃâ¬ÃÅMR Image Segmentation using a Power Transformation ApproachâÃâ¬ÃÂ, IEEE Transactions on Medical Imaging, Vol. 28, No. 6, pp. 894-905, June 2009.
- Julian Guerrero, Septimiu E. Salcudean, James A. McEwen, Bassam A. Masri, and Savvakis Nicolaou, âÃâ¬ÃÅReal-Time Vessel Segmentation and Tracking for Ultrasound Imaging ApplicationsâÃâ¬ÃÂ, IEEE Transactions on Medical Imaging, Vol. 26, No. 8, pp. 1079-1090, August 2007.
- M. Kass, A. Witkin, and D. Terzopoulos, âÃâ¬ÃÅSnakes: Active Contour ModelsâÃâ¬ÃÂ, IJCV, Vol. 1, Issue 4, pp. 321-331, 1988.
- M. Mignotte, and J. Meunier, âÃâ¬ÃÅA Multiscale Optimization Approach for the Dynamic Contour-based Boundary Detection IssuesâÃâ¬ÃÂ, Computerized Medical Imaging and Graphics, PERGAMON, Vol. 25, pp. 265-275, 2001.
- K. Padmapriya, and T. K. Bino, âÃâ¬ÃÅBoundary Detection using Edge Following Algorithm and Enhancement of the ImageâÃâ¬ÃÂ, International Conference on Computing and Control Engineering (ICCCE 2012), 12-13 April 2012.
- A. Santhosh Kumar, and J. Seetaram, âÃâ¬ÃÅMedical Images Boundary Detection using a New Novel Algorithm and Gradient FeaturesâÃâ¬ÃÂ, International Journal of Engineering Research and Technology (IJERT), Vol. 1, Issue 8, pp. 1-4, October 2012.
- S. Veeralakshmi, S. Vanitha Sivagami, V. Vimala Devi, and R. Udhaya, âÃâ¬ÃÅBoundary Exposure using Intensity and Texture Gradient FeaturesâÃâ¬ÃÂ, IOSR Journal of Computer Engineering (IOSRJCE), Vol. 8, Issue 1, pp. 28-33, Nov.-Dec. 2012.
- X. W. Zhang, J. Q. Song, M. R. Lyu, and S. J. Cai, âÃâ¬ÃÅExtraction of Karyocytes and their Components from Microscopic Bone Marrow Images based on Regional Color FeaturesâÃâ¬ÃÂ, Pattern Recognition, Vol. 37, No. 2, pp. 351-361, 2004.
|