ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
K.UMAKANTH1, H.R.SREEPAD2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
A novel isomeric polyimide/SiO2 hybrid material was successfully prepared through sol–gel technique, and its structure, thermal properties and nanoindenter properties were investigated. The researches on solubility and thermal properties of PEIPTES show that it can be used for modifying nano-SiO2 precursor. Then the PEIPTES solution and the nano-SiO2 precursor were mixed for 6 h to let the PEIPTES molecules react with the nano-SiO2 precursor, and modified nano-SiO2 precursor was obtained. Thermo gravimetric analyzer, dynamic mechanical thermal analyzer and nanoindenter XP was employed to detect the properties of the materials, the results demonstrated that isomeric polyimide/SiO2 hybrid material has much better thermal properties and nanoindenter properties than those of isomeric polyimide. The discussion covers solubility, thermal and dielectric properties, permeability and perm selectivity for gas separation, and rheology of isomeric polyimides. Several useful general rules have been found: i.e. the glass transition temperature of polyimides based on isomeric dianhydrides with a given diamine decreases in the order 3,3′->3,4′->4,4′-dianhydride if the polymers are of comparable molecular weight, whereas the thermal stability and the Tβ/Tg ratio (in absolute temperatures) increase in the order of 3,3′- <3,4′- <4,4′-dianhydride. Polyimides from 3,3′- or 3,4′-dianhydride have higher solubility than those from 4,4′-dianhydride. Polyimides from 3,4′-dianhydrides exhibit much lower melt viscosity than those from the other isomeric anhydrides. The dielectric constants of polyimides derived from m,m′- diamines are lower than those from p,p′-diamines. Polyimides based on 3,3′- or 3,4′-dianhydrides have higher permeability and slightly lower perm selectivity than polyimides based on 4,4′-dianhydrides.
Keywords |
| Isomeric , Properties, Sio2 ,Polyimide, Nano, Dianhydrides. Dielectric. |
INTRODUCTION |
| Polyimides (PI) have been widely used in the microelectronic industry for their excellent tensile strength and modulus, low thermal expansivity and dielectric constant, and good resistance to organic solvents.1 Some application properties, such as mechanical strength, require control or enhancement with PI. Most pure PI polymers are insoluble in organic sol-vents. Molecular chain modification by introducing a flexible linkage or a pendant group can make PI sol-uble.2 However, it is difficult to adjust PI thermo-physical properties by changing their chemical structure. On the other hand, the incorporation of inorganic materials like silica is a promising approach to enhance the thermal and mechanical properties of PI. |
| In the past decade, research has been focused on advance composite materials such as organic/inorganic composites,3–5 which combine the advantages of organic polymers with the benefits of inorganic materials. To improve the thermal stability and mechanical strength of polyimides, PI/silica composites have been under extensive study.6 –18 Beecroft et al.10 devel-oped transparent hybrids that showed improved hardness and modulus with increasing silica content. Zhu and coworkers13 prepared photosensitive PI/silica hybrids from a soluble PI. Many synthesis and processing methods have been developed for preparing organic/inorganic hybrid materials. The sol-gel process is the most common method.15,19,20 Wilkes and coworkers19 were among the first to exploit this concept by producing hybrids. The process consists of two steps: hydrolysis of inorganic alkoxides to produce hydroxyl groups, followed by polycondensation of the hydrolysis products and residual alkoxyl groups to form a three-dimensional network. The sol-gel process provides a method for preparing a variety of organic–inorganic hybrid materials at the molecular level. The in situ development of a three-dimensional cross linked inorganic network uses an organic precursor, such as tetraethoxy silane (TEOS).6,11,13,18 Many factors influence the kinetics of the hydrolysis and condensation reactions in the sol-gel process, which include pH, temperature, and the nature of solvent. |
| The great difference in the properties of polymer and silica materials can often cause phase separation. Decreasing the extent of phase separation (or increasing compatibility) can be accomplished by a variety of methods,15 as follows: (1) functionalizing the polymer chains at their ends, (2) selecting appropriate groups of polymers within the repeat units, (3) including comonomers that contain appropriate functional groups, or (4) adding a coupling agent to bond the PI chains and the inorganic oxide network. Mascia and Kioul7 produced PI/silica hybrids by the sol-gel route from solution mixtures of hydrolyzed TEOS and an aromatic polyamic acid. Compatibilization of the two components was achieved with the addition of a small amount of 3- glycidyloxypropyltrimethoxysilane (GPTMOS). Schrotter et al.9 prepared PI/silica hybrids by using various coupling agents to provide chemical bonding between the inorganic and the organic phase. They found that the properties of the organic side groups of the coupling agent are determinant on the properties of the final material. Chen and Iroh12 found that the presence of chemical bonds between PI and silica has a great effect on the properties of PI films, especially on their mechanical properties. Shang et al.16 studied the effect of coupling agent on the properties of PI/silica. Cornelius and Marand17 fabricated a series of PI/silica composites from a functionalized fluorinated PI and various methoxysilanes via a sol-gel process. The degrees of crosslinking between the silica structures and the PI matrices, as well as the morphology of the hybrid systems, were highly de-pendent on the type and content of the alkoxide employed. In this study, a coupling agent GPTMOS was chosen to increase the compatibility between PI and silica and to improve the mechanical properties of the hybrid materials. |
| Most of the PI/silica hybrids are prepared by a twostage sol-gel process: polyamic acid (PAA) is first synthesized; then hydrolyzed TEOS is added to proceed to the sol-gel process.12,16,18 The advantage of this procedure is that the uncyclized carboxyl groups can form hydrogen bonds with the acid hydrolyzed Si(OH)4 solution and disperse homogeneously within the polymer. However, the large amount of water released from the cyclization can break the molecular chains and deteriorate the properties of polymer. |
| In recent years, the invention of soluble polyimides has made it possible to prepare a PI/silica composite directly from a PI solution.17 In this study, we employed an approach of using the cyclized soluble polyimide as the polymer moiety. For the monomer synthesis of soluble polyimide, we selected diamine with a pendant phenyl hydroxyl group, 4,4 -diamino-4 - hydroxytriphenyl methane (DHTM).21 This functional group reacts with the coupling agent GPTMOS and enhances compatibility between PI and silica. The presence of hydroxyl groups in polyimide can alsoform hydrogen bonds with Si(OH)4 to achieve a ho-mogeneous dispersion. |
| Three methods to add silica nanoparticles into a polymer matrix were investigated: (1) charging acidic hydrolyzed TEOS solution into a polyimide solution, stirring to complete the reaction, then preparing the polyimide film; (2) adding a coupling agent, GPTMOS, to the above hydrolyzed TEOS solution and pouring this mixture into a polyimide solution16; (3) charging GPTMOS into a polyimide solution, stirring the mixture at 70°C for 24 h to react the epoxy group in GPTMOS with the hydroxyl group in polyimide, and forming a covalent bond. Then, the hydrolyzed TEOS solution was added to form a nanocomposite.22 We studied the effect of these three approaches on the thermal, transparent, and mechanical properties of polyimide/silica nanocomposites. |
EXPERIMENTAL SETUP |
Materials Used |
| Chemicals of high purity were obtained from various commercial sources, which consisted of pyromellitic dianhydride (PMDA; Chriskev, Leawood, KS), aniline (Janssen Chemical, Belgium), 4-hydroxybenzaldehyde (Acros), aniline hydrochloride (Acros), TEOS (Acros), GPTMOS (Acros), N-methyl-2-pyrrolidone (NMP; Tedia), ethanol (Tedia), and xylene (Tedia). NMP was purified by distillation under reduced pressure over calcium hydride and stored over 4 Å molecular sieves. Other organic solvents were purified by vacuum distillation. PMDA was dehydrated by drying under a vacuum at 170°C for 24 h. The other reagents were used as received. |
Synthesis |
| The reaction scheme is shown in Scheme 1. The reactor was preheated to 50°C and purged with nitrogen for 30 min. A mixture of 4-hydroxybenzaldehyde 61 g (0.5 mol), aniline 139.5 g (1.5 mol), and aniline hydrochloride 6.54 g (0.05 mol) was charged and stirred at reflux 120°C under a nitrogen flow for 3 h. After the com pletion of the reaction, the mixture was cooled and 100 mL ethanol was added. The entire mixture was then heated to 80°C to dissolve the lumpy material, cooled, and held overnight. Purple crystal precipitated out, was filtered off, and was recrystallized in ethanol. The amount of ethanol used was controlled by the solubility of DHTM at reflux ( 40 g/500 mL). Crude crystalline product was isolated and dried. The product was washed with ethanol a few times to remove the aniline residue and was washed with water to remove the salts. A pink powder product was obtained. Fur-ther recrystallization from ethanol (three times) pro-duced a purple needle crystalline product of DHTM. |
| Melting point (m.p.): 208°C. Yield: 40%. IR (KBr): 2860 cm 1 (C—H, aliphatic). 1H-NMR (DMSO-d6): 9.15 (s, OH, 1H), 6.9 – 6.4 (m, PhH, 12H), 5.04 (s, CH, 1H), 4.82 (s, NH2, 4H). Elemental analysis: C19H18N2O, Calcd.: (%) C, 78.60; H, 6.25; N, 9.65. Found (%): C, 78.2; H, 6.38; N, 9.44. |
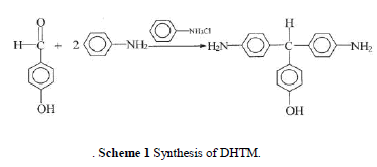 |
Synthesis of soluble polyimide |
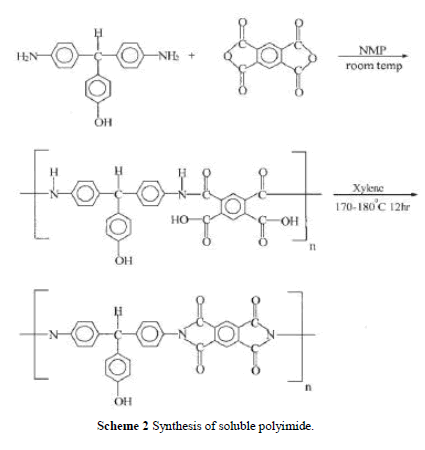
| Soluble polyimide was synthesized by reacting DHTM with PMDA as shown in Scheme 2. The reactor was purged with dry nitrogen for 30 min. DHTM 2.9 g (0.01 mol) and NMP was charged into the reactor (solid content 15%) and stirred until all diamine was dissolved in NMP. PMDA 2.18 g (0.01 mol) was charged in several portions and stirred at room tem-perature for 2–3 h to obtain a viscous polyamic acid solution. Xylene (15 mL) was added and the mixture was heated to 170 –180°C at reflux for 12 h until the water was azeotropically distilled off via a Dean–Stark trap. Heating was continued to distill off the residual xylene. After completion of polymerization, the viscous polyimide solution was cooled and dissolved in an adequate amount of NMP, and the polymer solution was ready for the preparation of PI/silica nano-composites. Polymerization yield was 85%. The inherent viscosity of PI was 0.85 dL/g measured at a con-centration of 0.5 g/dL with NMP as the solvent at 30°C. IR: 3490 cm 1 (—OH), 2860 (C—H, aliphatic), 1778, 1726 (CAO), 726 (C—N, tertiary). 1H-NMR (DMSO-d6) 9.38 (s, OH, 1H), 8.35– 6.74 (m, PhH, 14H), 5.69 (s, CH, 1H). |
 |
Preparation |
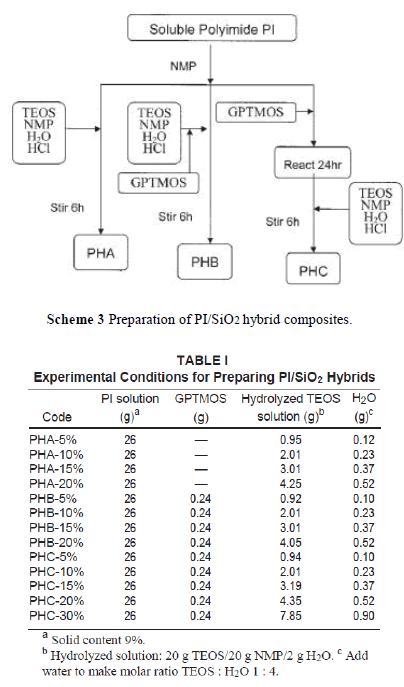
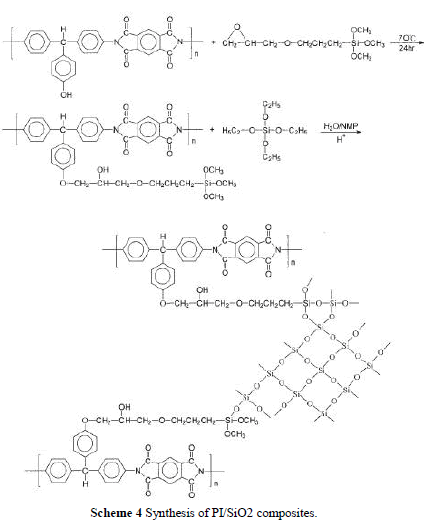
| In this study, we prepared the PI/SiO2 hybrid nanocomposite by a sol-gel process directly from the PI solution. There are three ways to add silica nanoparticles into the polymer matrix as depicted in Scheme 3. PHA was obtained by charging hydrolyzed TEOS solution into a PI/NMP solution. PHB was formed by adding the cou pling agent, GPTMOS, to the hydrolyzed TEOS solution, and then this mixture was poured into a PI solution. Last, GPTMOS was charged into a PI solution and stirred at 70°C for 24 h to complete the reaction. Then, the hydrolyzed TEOS solution was added and stirred for 6 h to produce a hybrid PHC solution. The amount of hydrolyzed TEOS solution and GPTMOS used are shown in Table I. |
| The hybrid polymer solutions were spread on a glass plate by using a spin-coater to control the film thickness at 10 –15 m for IR and UV–Vis analysis and at 50 – 60 m for Instron analysis. The films were thermally dried at 50°C to remove most of the solvent and stripped from the glass. Then, the films were fixed in a film casting apparatus and heated in a vacuum oven at 170 –180°C for 24 h to remove all solvents. |
Measurements |
| Fourier transfer infrared (FTIR) spectra were recorded on a Bio-Rad FTS-40A spectrometer. 1H-NMR spectra were performed on a Bruker AMX- 400 spectrometer with dimethylsulfoxide (DMSO-d6) as the solvent. The morphologies of the fracture surfaces of the hybrid materials were observed with a Hitachi S-4100 scanning electron microscopy (SEM). The inherent viscosities were determined by using a Ubbelohde viscometer (Schott Gerate AVS310) at 30°C. Molecular weight was determined by gel permeation chromatography (GPC) with polystyrene calibration by using a Perkin–Elmer series 200 HPLC system equipped with Jordi Gel DVB column at 35°C in THF. An Instron universal tester model 4467 was used to study the stress–strain behavior of the samples. The load cell used was 5 kg and the crosshead rate was 5 mm/min. Measurements were performed with film specimens (1.35 cm wide, 6 cm long, and 50 – 60 m thick). The gauge length was 2 cm. UV–Vis spectra were measured on a Perkin–Elmer Lambda 10 UV–Vis spectrophotometer at room temperature. The film thickness was 10 –15 m. For the measurement of moisture ab-sorption rates, specimens were exposed to water for 48 h and the weight difference was calculated after wiping off the water on the surface. Thermogravimetric analysis (TGA) was performed with a Perkin– Elmer TGA-7 thermal analyzer at a heating rate of 20°C/min in nitrogen within the temperature range of 30 – 800°C. Differential scanning calorimetry (DSC) data were obtained from a Perkin– Elmer DSC-7. Samples of 5 mg were sealed in hermetic aluminum pans and scanned in the calorimeter at a heating rate of 10°C/min in the range of 30 – 400°C under N2 atmosphere. The glass-transition temperature (Tg) values were taken as the change of the specific heat in the heat flow curves. Dynamic mechanical analysis (DMA) was performed on a Perkin–Elmer DMA-7 thermal analyzer system in tensile mode at a frequency of 1 Hz from 60 to 400°C. A sample 15 mm long and 5 mm wide was used. |
 |
RESULTS AND DISCUSSION |
Syntheses of Monomer and Polymer |
| Monomer DHTM was synthesized from 4-hydroxybenzaldehyde and aniline in the presence of an acid catalyst (aniline hydrochloride). The protonation of the aldehyde group produced a positively charged carbon and the resonance effect of aniline activated an amino group to form a nucleophilic group in the para position. DHTM was then produced by a nucleophilic reaction. The low yield was due to the formation of ortho isomer byproduct from aniline and aldehyde. Because excess aniline was used, the product and byproduct were mixed as a very viscous material. Isolation of DHTM required tedious steps of purifica-tion.23 |
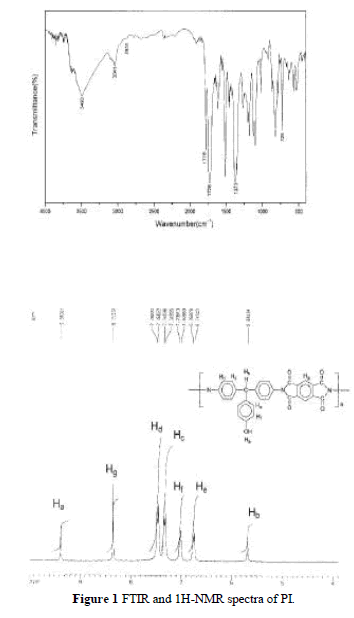
| Soluble PI was synthesized including a ring-open-ing polyaddition of diamine DHTM with dianhydride PMDA as shown in Scheme 2, followed by cyclodehy dration of imidodicarboxylic acid by means of xylene– water azeotropic distillation. Molecular weight, Mw, of the soluble polyimide was measured by using GPC and the chromatogram indicated the Mw was 64,000. The FTIR spectrum of the PI is shown in Figure 1(a). It exhibits characteristic imide group absorptions at 1778 and 1726 cm 1 (typical of imide carbonyl asymmetrical and symmetrical stretch), and at 726 cm 1 because of the tertiary amine structure. Figure 1(b) shows the 1HNMR spectrum. The characteristic peaks correlate well with the proposed structure. |
Preparation of nanocomposite |
| Because TEOS was insoluble in water, a cosolvent was needed to prepare a hydrolyzed TEOS solution. If the sol-gel reaction was carried out in PAA solution, ethanol or methanol was often used as cosolvent. However, when TEOS ethanol solution was added in a polymer solution, the polymer precipitated out and became an opaque solution. To over-come this problem, we use NMP as the cosolvent. NMP (20 g) can dissolve 20 g of TEOS in 2 g of water, even with the addition of 0.01 wt % HCl. To make a molar ratio of TEOS : H2O equal to 1 : 4, additional water was needed. The incorporation of a coupling agent, GPTMOS, was to increase compatibility between PI and silica and resulted in improv-ing the mechanical properties of the hybrid materi-als. In the preparation of hybrid PHB, the epoxy group in GPTMOS was acid hydrolyzed to form hydroxyl groups that can form hydrogen bonds with the carbonyl groups in polyimide, which led to increased compatibility. For the preparation of hybrid PHC, as shown in Scheme 4, the epoxy group in GPTMOS reacted with the hydroxyl group in polyimide to form a covalent bond. Then, the other end of GPTMOS was hydrolyzed and reacted with TEOS to form a composite network. |
 |
 |
FTIR characterization of the PI/SiO2 hybrid films |
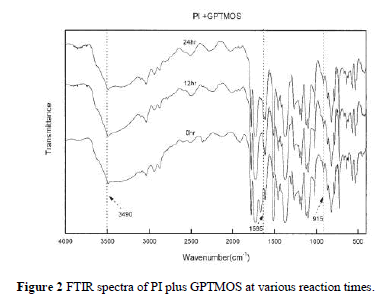
| Figure 2 demonstrates the FTIR spectra of the reaction between PI and GPTMOS in the preparation of PHC. The figure displays the change of the characteristic peak of the epoxy group of GPTMOS at 915 cm 1 and the formation of the ether group at 1595 cm 1. The ring-opening reaction of GPTMOS caused the peak at 915 cm 1 to disappear gradually and the formation of ether functional group with PI caused the ether peak to appear significantly with time. The wide absorption band around 3490 cm 1 corresponding to the stretch-ing vibration of a hydroxyl group also slightly de-creased over time. The result confirms that PI reacted with GPTMOS at various times to some extent and indicates that bonding groups in PHC are not all ether covalent bonds. |
 |
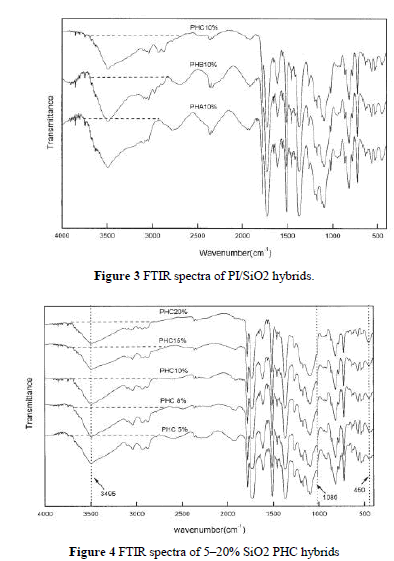
| Figure 3 shows FTIR spectra of three PI/SiO2 hybrids, PHA, PHB, and PHC, each with 10 wt % silica content. The characteristic peak of the hydroxyl group in PHC was weaker than that in PHB and PHA. PHA has the strongest peak. This indicates that, with the existence of a coupling agent, the hydroxyl group in PI can react with the epoxy group of GPTMOS at high temperatures via condensation. |
| shows the IR spectra of PHC with various amounts of silica. All the films have the characteristic absorption peaks of imido groups and Si—O. Because of the presence of a strong absorption at 1090 cm 1 from C—O, the characteristic peak of Si—O stretching vibrations (near 1080 cm 1) was not obvious, but we found that the band of Si—O bending vibration at 454 cm 1 increased with greater silica content. The wide band at 3495 cm 1, corresponding to the stretching vibration of C—OH, decreased with increasing silica content. This may have been related to the decrease of hydroxyl concentration because of the condensation between silanol and the hydroxyl group.18 It is evident that, even without a coupling agent, PI with hydroxyl group is compatible with silica to some extent as in the case of PHA. |
 |
Mechanical properties of the hybrid films |
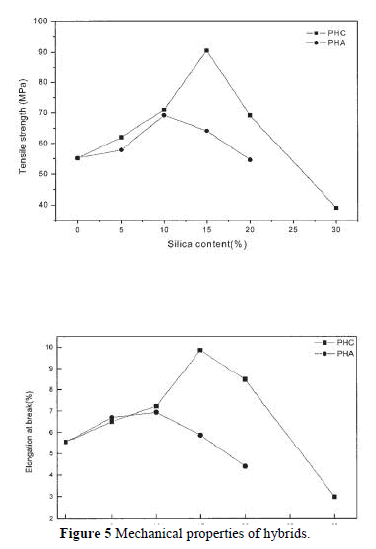
| The compatibility between PI and silica strongly affects the thermal, mechanical, and optical properties of PI. The influence of silica content on the mechanical properties is shown in Figure 5. The elongation at break and the tensile strength increase with the addition of silica up to 10 wt % for PHA and PHC. Then, a fast reduction of tensile strength or elongation at break was observed for PHA, which was related to the phase separation. It was remarkable to observe a continuous increase of the tensile strength and elongation at break for PHC when 15% silica was added; the tensile strength and elongation at break reached 91 MPa and 9.86%, respectively. This increase in tensile strength and elongation can be attributed to the improved interaction between the PI matrix and the silica resulting from the chemical bonds introduced by the coupling agent. The increase in tensile strength resulting from the development of a cocontinuous phase morphology (as will discussed later) with the addition of GPTMOS coupling agent can obviously be attributed to a more efficient stress transfer mechanism between the two components.7 Both the tensile strength and the elongation at break decrease when the silica content exceeds 15 wt %. These phenomena might be the distinctive features of a nanocomposite. The test results for the PI/silica nanocomposites are comparable with other published results. |
 |
Morphological properties |
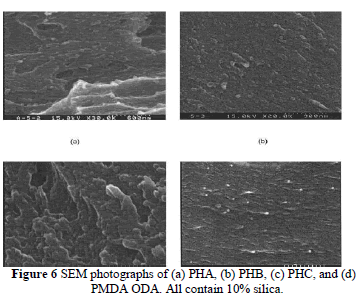
| The morphology of the fractured surfaces was elucidated by SEM to investigate the distribution of silica and microphase separation in the hybrid matrix. The SEM in Figure 6(a– c) compares the fractured surface of hybrid films (all containing 10 wt % silica) made by various methods. They all showed good miscibility between polymer and silica phases, especially for PHC (which was made with the reaction of coupling agent, GPTMOS). When the coupling agent was added, the phase separation improved. From the scale, one can see the particle sizes within the hybrid films made by sol-gel process is 50 –100 nm. This demonstrates that nanocomposites can be made with the current process. This phenomenon reveals a morphological transformation from a dispersed particle microstructure to a fine interconnected or cocontinuous phase. The presence of a hydroxyl group condensed with silanol at high temperature contributed to the miscibility of this cocontinuous phase. In Figure 6(d), the dispersed silica particles can be seen as white beads from a SEM photograph on the fractured surface of the hybrid film (containing 10 wt % silica) made with PI without hydroxyl groups [synthesized from PMDA and 4,4 - oxydianiline (ODA)]. This again demonstrates the contribution of hydroxyl groups to the compatibility of PI and SiO2. |
 |
Transmittance |
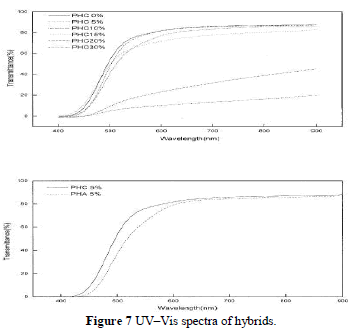
| Most polyimide–silica hybrids are transparent, because the nanoparticle diameter is less than the wavelength of visible light. When hybrids are produced with higher silica contents ( 15%), phase separation becomes evident, which leads to an opaque appearance as shown in Figure 7(a). PHA and PHC (all with 5% silica) are com-pared in Figure 7(b), which depicts the contribution of coupling agent to the transmittance. |
 |
Water absorption |
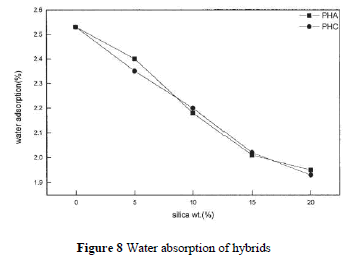
| PI contains carbonyl groups and has unshared electron pairs, so it can easily form hydrogen bonds with water. Soluble PI, with hydroxyl groups, has higher water absorbance. One can expect that silica hybrid composites have less moisture absorption. Figure 8 depicts the water absorption rates that decrease with increasing silica contents for PHA and PHC. However, the difference between PHA and PHC is not significant. This indicates that the addition of a coupling agent has no effect on the water absorption rate. |
 |
Thermal properties |
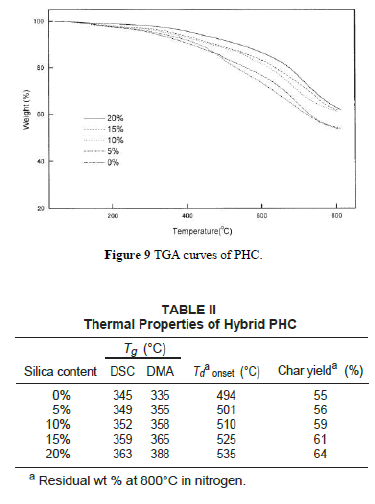
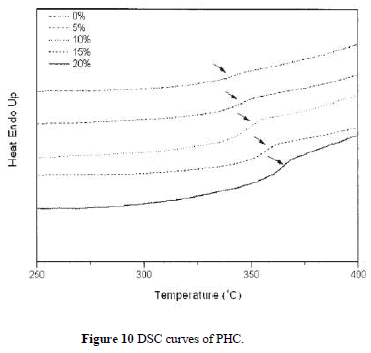
| Figure 9 depicts TGA curves of the hybrid composite PHC with various silica contents. The thermal decomposition temperature (under N2 atmosphere) increases with increasing silica content as listed in Table II. The char yields of the hybrid materials increase with greater amounts of silica. Figure 10 shows the DSC traces of the hybrid PHC with different silica contents. The Tg thus obtained are listed in Table II. It can be seen that the Tg of hybrids increases with increasing silica content. These results indicate that the coupling agent strengthens the interaction between the organic polymer matrix and the inorganic particles, which causes an increased restricting strength of silica on PI molecules and hence increases the Tg of the composite. These data are consistent with the results by DMA as also shown in Table II. |
 |
CONCLUSION |
| Soluble polyimide was synthesized via a procedure that included ring-opening polyaddition of diamine DHTM (synthesized from 4-hydroxybenzaldehyde and aniline) to dianhydride PMDA, followed by cyclodehydration via xylene–water azeotropic distillation. |
| The introduction of a hydroxyl group to PI resulted in the improved solubility of PI and increased the compatibility of PI with silica. This was due to the partial condensation between the hydroxyl group and silanol at high temperatures. The incorporation of a coupling agent, GPTMOS, was shown to improve compatibility between polyimide and silica, which improved the mechanical properties of the hybrid materials. As in the preparation of the hybrid PHC, the epoxy group in GPTMOS reacted with the hydroxyl group in polyimide to form a covalent bond. The other end of GPTMOS hydrolyzed and reacted with TEOS to form a composite network. The increased silica content reduced the hydroxyl characteristic band in FTIR. This indicated that reaction between silanol and the epoxy group with the hydroxyl group occurs at high temperatures. A comparison of the hybrids PHA and PHC determined that their optimum SiO2 content was 10 and 15%, respectively. PHC had better mechanical property, higher optical transparency, and better thermal properties than PHA. This elucidates the contribution of the coupling agent, GPTMOS, to the improved compatibility and physical properties of hybrid nanocomposite. |
 |
References |
|