e-ISSN: 2319-9849
e-ISSN: 2319-9849
Laboratory of Applied Chemistry: Heterocycles, Lipids and Polymers, Faculty of Sciences, University of Sfax, BP 802, 3000 Sfax, Tunisia
Received date: 27/09/2017 Accepted date: 10/10/2017 Published date: 29/10/20176
Visit for more related articles at Research & Reviews: Journal of Chemistry
This review article summarizes recent developments and trends in the application of imidates as precursors for the syntheses of heterocyclic systems such as pyrimidine derivatives, quinoline-oxadiazole, triazoles and triazines Also described are less usual examples of applications in which they and their analogies react as monofunctional precursors, or where they have been used as sources of a nitrile carbon atom Their chemical and/or biological properties and potential applications are discussed, along with those of the derived heterocycles
Substituted Imidates; Chemistry; Anti-microbial Activities
This review covers the type of compounds variously described as imino ethers, imido esters, imidic esters, imidoatee, and imidates as well as their N-substituted derivatives The general structure of this compound is in Figure 1.
Throughout this article, it is proposed that compounds of this class should be named after the parent imidic acids and termed imidates: thus, compound Ia is methyl propionimidate, Ib is phenyl N-phenylbenz-thioimidate hydrochloride, and Ic is ethyl N-(cyano-N'-methylcnrbamoylmethyl) formimidate (Figure 2).
In other hand, There are several methods to form an imidate such as the Pinner reaction (acid-catalyzed alcoholysis of a nitrile) [1] synthesis from ortho-esters [2] or carbonyl compounds, the most common being direct alkylation of amides [3] The latter method, however, has an intrinsic issue relating to the competition between N- and O-alkylation N-Alkylation is commonly achieved under basic conditions (NaH, LHMDS, K2CO3) in polar solvents, using an alkyl halide, although the O-alkylation by-product is often observed, due to equilibration of the amide anion [4]. When O-alkylation is desired there are several possibilities, including treating an amide with dimethylsulfate, [5] diazomethane [6] or trialkyloxonium tetrafluoroborates (Meerwein’s reagent) [7], most often in combination with a hindered base, such as iPr2EtN [8-10].
In addition, the diverse biological activities reported for many derivatives of imidates have also drawn the attention of biochemists in the last decade The literature covering the chemistry of iminoethers derivatives has been limited However, a review of the chemistry and reactions of iminoethers was published [11]. The main objective of the present survey is to provide a comprehensive account of the synthetic utility of imidates or imidate N-fonctionnilazed in building various organic heterocycles and to highlight their potential in evolving better chemotherapeutic agents.
Synthesis
Synthesis of unsubstituted imidates
Imidates can be synthesized by numerous methods Many of these synthesis can also be applied to different substituted imdates derivatives simply by varying the functional groups on the reactants Several approaches are available for synthesis of imidates as, Pinner, Nef synthesis, hydratation, and alkylation Details of the synthetic procedures are given below.
Pinner and Klein synthesis: In 1877 Pinner and Klein discovered the proton-induced imidate syntheses [12,13]. They passed anhydrous gaseous hydrogen chloride through a mixture of an alcohol and benzonitrile A crystalline product precipitated, which they identified as an imidate hydrochloride (Scheme 1) Best results in the Pinner reaction are obtained Best results in the Pinner reaction are obtained with primary or secondary alcohols and aliphatic or aromatic nitriles.
A plausible mechanism (Scheme 2) starts with a protonation of the nitrile by the strong acid hydrogen chloride leading to a highly activated nitrilium cation, which can be attacked by the alcohol component Proton transfer (P.T.) yields the imidate hydrochloride [14].
Nef synthesis: The reaction was discovered by Nef in 1895 in his work with cyanogen This method reported the synthesis of imidates in basic medium in the presence of a nitrile on an alcohol and an alkoxide (Scheme 3) [15].
This method, even if it gives best results with aliphatic group, the yields are generally low, since in the basic medium the imidate can restore the starting nitrile.
Marshall and Acree synthesis: Marshall and Acree [16] in particular determined the position of equilibrium in the reaction of several nitriles with ethanol in the presence of sodium ethoxide at 25° Their studies established that the reaction wap alkoxidecatalyzed and that imidate formation was promoted by the presence of electron-attracting groups in the nitrile Moreover, this work demonstrated that the equilibrium constants for the reactions of several common nitriles were sufficiently large to be useful [17] Despite these promising early results, virtually no further use had been made of this reaction until the present work was undertaken [18-24] We have now extended somewhat the basic quantitative studies of Marshall and Acree to include consideration of certain preparatively important factors and have studied a broader range of nitriles including several of special significance in our concurrent work Our results reaffirm that many electronegatively substituted aliphatic and aromatic nitriles may be converted to imidates extremely easily in useful yield by base-catalyzed reaction with a lower alcohol This paper includes illustrations of the practical use of the process for the preparation of a number of interesting imidates The equilibrium conversion of nitrile to imidate in methanol at 25° in the presence of a catalytic amount of sodium methoxide [25] was determined for a wide variety of nitriles The results obtained in those cases where a reaction could be detected are presented in Scheme 4.
Although most of the data are self-explanatory, a few unusual results are discussed below:
(a) The equilibrium mixture obtained with succinonitrile appeared to contain approximately equivalent amounts of methyl 3-cyanopropionimidate (I) and the cyclic structure (II), possibly in equilibrium with each other
Acid hydrolysis of the reaction mixture in the course of measuring the extent of imidate formation produced an approximately equimolar mixture of a weak and a strong base (Schemes 5-10). These are presumed to be ammonia from I and 3-carbomethoxypropionamidine from II [26].
(b) The equilibrium conversion of 108y0 for terephthalonitrile is consistent for approximately 70-80% conversion to the monoimidate due to the strong activation of a pcyano substituent in ben-zonitrile plus 30-40 q;b diimidate due to the moderate activation of a pcarboximidate group.
(c) The conversion shown for cyanomethyltriethylammonium chloride was obtained with potassium cyanide as catalyst When the more basic sodium methoxide was used, the indicated maximum conversion (73%) was reached in ninety minutes Within a few hours, however, the apparent conversion dropped to 61% This is consistent with the proposition that the alkoxide is destroyed with an equivalent amount of the imidate by the Hofmann dégradation.
Synthesis via Aza-Claisen Rearrangements: A novel thermal 3-aza-Claisen rearrangement of N-allyl ynamides for the synthesis of a-allyl imidates is described by DeKorver et al. [27], We found that heating of ynamides 11 in alcoholic solvents in the presence of 4 Å molecular sieves led to imidates 12 in moderate to excellent yields.
Synthesis of N-substituted Imidates
Pinner's reaction in 1892 [12] is the first synthesis of N-acylated and N-ethoxycarbonylated imidates Indeed, the action of the simple imidates with the acid halides or with the ethyl chloroformate in the presence of a base (triethylamine or pyridine) directly gives the target compounds.
The condensation of NH2-structured compounds with orthoesters, which has been the subject of several methods of synthesis of N-functionalized imidates reported by several authors [28] This reaction requires an acid catalyst.
A simple and reliable protocol for regioselective O-alkylation of amides provides the corresponding imidates Its scope and efficiency is demonstrated on a number of substrates [29].
Imidate 21 was obtained by condensation of 5-amino pyrazole-4-carbonitrile 22 with triethyl orthoformate in the presence of acetic acid [30].
Chemical reactivity of N-substituted imidates
Various transformations are possible with the imidate hydrochlorides: Hydrolysis at low pH leads to carboxylic esters, where basic hydrolysis yields imidates Reaction with amines furnishes amidinium compounds and the reaction with alcohols A less frequently used pyrolysis leads to carboxamides (Scheme 11) [31-33]. The high toxicity and the laborious handling of gaseous hydrogen chloride are further drawbacks of this reaction Nevertheless, milder protocols have developed over the decades: Luo and Jeevanandam used trimethylsilyl chloride (TMSCl) and ethanol for an in-situ generation of hydrogen chloride [34] Watanabe et al. reported on a Pinner reaction with a 4 N hydrogen chloride solution in cyclopentyl methyl ether (CPME) [35]. An ionic liquid based on a sulfonic acid was used by Jiang et al. [36] where this method has only been applied to aliphatic nitriles A transition metalcatalyzed Pinner reaction using dihydridotetrakis(triphenylphosphano)ruthenium ([RuH2(PPh3)4]) as catalyst has been applied to aliphatic nitriles and alcohols and was similarly used for intramolecular reactions [37] Schaefer and al reported a base catalyzed Pinner reaction, which gave only poor yields because of the setting of an equilibrium [38] While developing a total synthesis of altenuic acid II [39,40] we observed the reaction of an aliphatic hydroxy group with acetonitrile in the presence of two equivalents of hafnium triflate [Hf(OTf)4] yielding the respective acetate A detailed investigation on this reaction is reported in this article [40].
Synthesis of triazoles: N-functionalized imidates have been widely used in the synthesis of triazoles [37-44] One of the simples routes involving the condensation of N-acylated imidates with hydrazines [41] is shown in Scheme 12.
Recently, M'hamed et al. [42] used N-functionalized imidates as a basic precursor for the synthesis of these same triazole frameworks (Scheme 13). This reaction involves these reagents with 3-hydrazino-2- (N, N-dialkylaminomethyl) propanenitriles under reflux of methanol.
Synthesis of triazines: In 1988, Kaddachi et al. [43] could show that N-acylated and N-ethoxycarbonylated imidates react with 3-amino-1,2,4-triazoles to give after prolonged heating to triazines according to Scheme 14.
In a recent study carried out in our laboratory by Chabchoub et al. [44], new triazolotriazines have been obtained by the action of 5-amino-1,2,4-triazoles on N-ethoxycarbonylated imidates (Scheme 15-20).
Another approach [45], has been extensively utilized in recent years for the synthesis of pyrazolo-triazine by condensation of N-acyl-imidates 45 with compound 44.
Synthesis of quinoline-1,2,4-oxadiazole: The condensation of amidoxime was heated under reflux for 5 min with 3 ml of acetic anhydride. The product being solidify on cooling The solid product was filtered off and recrystallized from ethanol, under heating condition as shown in the following mechanism [46].
Synthesis of benzoxazole : lmidates or salts of imidates of carboxylic acids are known to be convenient synthones in the synthesis of 2-substituted benzoxazole when it was reacted with various amines [47].
Synthesis of pyrimidinone: Mouna et al. prepared the tetrahydropyrimidine by stirring an equimolar amounts of ethyl N-ethoxycarbonylbenzimidate 54 with cyanoacetanilide derivatives 55, under basic medium [48].
Synthesis of acétamido-2-cyano-N,3-dihenylacrylamide: Similar syntheses have been extensively employed Under the same experimental conditions, Mouna et al. [49] have described the synthesis of acétamido-2-cyano-N,3-dihenylacrylamide which were prepared from compound 55 with ethyl N-acetylbenzimidate 57 in the presence of sodium ethanoate.
Synthesis of pyrimidin-2(1H)-ylidene)benzo[d]oxazole: In other hand, Reaction of 59 with ethyl cyanoacetate gave 2,3-dihydropyrimidin-4(1H)-ones 60 (Scheme 21). Their reaction mechanism was proceeding via condensation reaction between amino group and ester group with elimination of ethanol molecule followed by nucleophilic addition of the amino group on cyano group Similarly, a nucleophilic addition of the two amino of guanidyl group in compounds 59 to the two cyano groups in malononitrile afforded the corresponding hydropyrimidines 67 the 6-hydroxy-2,3-dihydropyrimidin-4(1H)-ones 67-70 were synthesized via reaction of 59 with ethyl acetoacetate, ethyl benzoylacetate or diethyl malonate in presence of the benign catalyst glacial acetic acid [50].
Synthesis of pyrazolo[3,4-d]pyrimidin-4-amine: It seemed of interest to react Imidate 71 with a series of amines In this case, we considered that the presence of amidine moiety may ensure the possibility of closure of the pyrimidine ring, resulting in novel derivatives of pyrazolo[3,4-d]pyrimidine of significant biological interest since such compounds are substituted analogues of the well-known drug allopurinol We selected some aromatic and aliphatic primary amines, the more basic ammonia and hydroxylamine hydrochloride, to study their reactions with the imidate [51].
The imidate 71 reacted with both their electrophilic sites with aliphatic amines to yield the new pyrazolopyrimidines type 73 in two steps In the first step, the condensation of 71 with aliphatic amines in ethanol in the presence of a catalytic amount of acetic acid led to the intermediate by the nucleophilic attack of the NH2 motif on imidic carbon In the second step, the isolable amidine 74 was heated in toluene in the presence of a few drops of piperidine to provide the novel pyrazolopyrimidines 73 via Dimroth rearrangement (Scheme 22) [52,53].
Biological Activities
Imidate derivatives and related heterocycles moieties have generated recent interest due to their interesting biological and pharmaceutical activities
In fact, the biological potential of imidate derivatives has been investigated in a few cases and important biological activities have been observed For example, gossylic iminolactone (GIL, I) has been found to exhibit anti-HIV activity (Figure 3) [54].
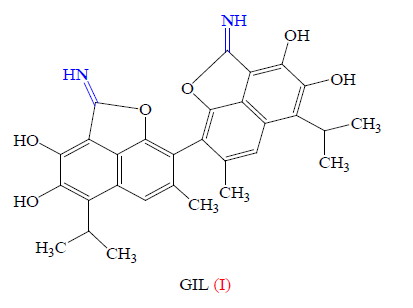
Furthermore, Hegde and col reported several cyclic imidate derivatives II of 5- amino-2,6-bis(polyfluoroalkyl)pyridine-3- carboxylates having interesting herbicidal activities [55].
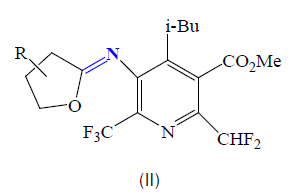
They have been also, reported that imidates derivatives to be active against nociceptive and Antipyretic activity [56]. Furthermore, They have also been used for their anti-inflammatory [57] and anti-bacterial [58-64].
The present survey has clearly demonstrated that Imidate may be successfully used to synthesize a wide variety of heterocycles of academic and pharmaceutical interest. Moreover, in general, the desired compounds may be obtained in a single step with high yield.