e-ISSN: 2320-7949 and p-ISSN: 2322-0090
e-ISSN: 2320-7949 and p-ISSN: 2322-0090
1Department of Oral and Maxillofacial pathology and Microbiology, Vasantdada Patil Dental College and Hospital, Kavalapur, sangli, Maharashtra, India
2MD Microbiology, Department of General Pathology and Microbiology, Vasantdada Patil Dental College and Hospital, India
Received Date: 17/06/2016; Accepted Date: 12/09/2016; Published Date: 19/09/2016
Visit for more related articles at Research & Reviews: Journal of Dental Sciences
Background: Dental caries is a multifactorial disease and the important etiologic factors include streptococcus mutans and lactobacilli counts, salivary flow rate, buffering capacity and past caries experience. It is also modified by factors like type of diet, oral hygiene practices, uses of fluoride and other preventive measures. Although immense research has been done on all variables affecting dental caries, models having better predictive power in caries risk assessment have not been validated among Indian population. Hence, the purpose of the present study was to evaluate whether salivary counts of S. mutans combined with measurement of salivary flow rate and buffer effect can be used for diagnostic and predictive purpose in cariology. Materials and methods: The study was conducted amongst 50 school children aged between 9 to 12 years and were divided in two groups based on their DMFT, def scores as caries active and caries free respectively with 25subjects each. Collected saliva samples were processed to analyse salivary flow rate, buffering capacity and Streptococcus mutans count on Mitis Salivarius Kanamycin Bacitracin (MSKB) selective growth medium. Results: Statistically significant difference was observed on quantitative and qualitative comparison of salivary flow rate, buffering capacity and log Streptococcus mutans CFU counts in saliva of dental caries active and caries free group with p value at 0.00001 (p<0.05). Conclusion: The mutans selective growth medium, MSKB is an excellent diagnostic medium for growth of Streptococcus mutans. Salivary variables like flow rate and buffering capacity can serve as a valuable diagnostic tool to assess caries experience and predict caries risk.
Dental caries, Lactobacilli, Saliva, Salivary pH, Streptococcus mutans, MSKB media
Dental caries is an irreversible microbial disease of the calcified tissues of the teeth, characterized by demineralization of the inorganic portion and destruction of the organic substance of the tooth, which often leads to cavitation. Among the oral diseases, dental caries is the most common chronic disease of mankind. It affects persons of both sexes in all races, all socioeconomic strata and any age group. As children reach school age, they have an increasing incidence of carious lesions because of change in dietary habits which includes refined carbohydrates and sweeteners [1]. Dental caries is a multi-factorial disease and the important etiologic factors includes Streptococcus mutans (S. mutans) and lactobacilli counts, salivary flow rate, buffering capacity and past caries experience [2]. It also modified by factors like type of diet, oral hygiene practices, uses of fluoride and other preventive measures [3,4]. The factors related to the development of dental caries are extremely relevant in the disease process. The microorganisms with cariogenic capacity do not determine the presence of dental caries. It is necessary to have suitable substrates and physiological conditions in the host to allow implantation and survival of these micro-organisms in order to facilitate the development of caries.
In India, children comprise 40% of a rapidly growing population. The prevalence of dental caries varies from 33.7% - 90% in children and is increasing at an alarming rate [5] and ranks amongst the most common human diseases. S. mutans and lactobacilli have been extensively studied and have shown to play an important role in the aetiology of dental caries [6]. S. mutans has been strongly associated with initiation, while lactobacillus has been connected with progression of carious lesions [7]. Being acidogenic and aciduric microorganism, the colonization of S. mutans is considered to be the main cause of dental caries [8]. S. mutans level in saliva has been shown to be one way of predicting caries activity and therefore several tests to estimate caries risks based on mutans levels have been developed. Common among all these tests is the use of growth medium supplemented with sucrose and bacitracin for selective recovery of S. mutans from total saliva. A new medium MSKB composed of salivarius agar base, sorbitol, kanamycin sulphate and bacitracin has been developed which is a more selective growth medium and appears to be better suited for use in caries diagnostic tests than the traditional MSB medium. The new MSKB medium is more selective for mutans as it eliminates false positive results caused by visual interpretation of non mutans as mutans [9].
Salivary variables like flow rate, buffering capacity and anti-microbial activity have been recognized as having the ability to reduce the incidence of dental caries. Although immense research has been done on all variables affecting dental caries, models having better predictive power in caries risk assessment have not been validated among Indian population [10]. Thus, there is need of combination of diagnostic tests to target high risk caries groups. Hence, the purpose of the present study was to evaluate whether salivary counts of S. mutans combined with measurement of salivary flow rate and buffer effect can be used for diagnostic and predictive purpose in cariology.
To evaluate and compare the relationship between salivary S. mutans count, salivary flow rate and buffering capacity in caries active and caries free school children aged between 9-12 years and to determine the efficacy of MSKB as a selective medium for isolation of S. mutans.
Institutional Ethics Committee clearance was obtained prior to commencement of study. The study was conducted amongst fifty school children aged between 9 to 12 years. The age group of 9 – 12 years was selected for the study for the following reason:
9 – 12 years is one of the index age groups as recommended by the World Health Organization.
It is the global monitoring age for international comparisons and monitoring of disease trends.
It is one of the very ages or risk ages to be consisted for caries risk assessment for screening in schools [11].
As this was a comparative study where in the salivary variables were compared to the caries experience of the subjects (caries active and caries free), the caries experience groups were made to contain an equal number of subjects (n=25) in each group. The caries status of each child was scored by using DMFT, def indices and assigned into two groups based on their DMFT, def scores i.e. DMFT, def>0 for caries active and DMFT, def=0 for caries free. The cases were then arranged in two groups; group 1: caries active (n=25) and group 2: caries free (n=25). Informed written consent was recorded from all subjects before collection of saliva samples. Children who were severely ill, having difficulty in opening the mouth, who had taken antibiotics in the last month and those wearing orthodontic appliances were excluded from the study. The study was carried out using a specific pretested proforma consisting of two parts.
First part consisted of information of school children regarding age, sex and clinical oral examination and the second part consisted of DMFT, def indices and salivary analysis.
Saliva samples were collected between 10 am -11 am to control the circadian variations. Participating children were instructed not to eat or drink anything for at least two hour before the collection of saliva samples. Children were asked to rinse their mouth with water thoroughly 10 minutes before collection of saliva to avoid the contamination of food debris. Children were made to swallow the pre-existing saliva in order to clear the mouth of any residual un- stimulated saliva. For collection of stimulated saliva, the children were asked to chew pieces of sterile chewable paraffin wax pellets (0.5x0.5 cm) and instructed to let saliva collect in the floor of mouth without swallowing it for at least 1 minute. Saliva was then withdrawn with the help of sterile syringe. This procedure was continued for a period of 5 minutes and stimulated saliva was collected. The saliva sample of all the children was identified by a code number during the period of sample collection and processing. After measuring the flow rate of stimulated saliva, the sample was carried in a polystyrene tube containing thioglycolate broth transport media (Hi media) to the laboratory for further analysis of buffering capacity and S. mutans count.
The flow rate of stimulated saliva was determined by the following formula [12]:
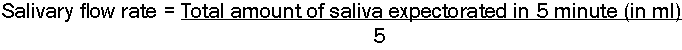
The salivary flow rate was expressed in ml/minute and scored as follows:
Score 0: >0.7 ml/minute; Score 1: 0.3 – 0.7 ml/minute and Score 2: <0.3 ml/minute.
The estimation of buffering capacity was carried out as per the method described by Ericsson [12,13] modified for smaller volumes. 0.5 ml of saliva was added to 1.5 ml of 5 moles/1 HCL. The mixture was vigorously shaken and then centrifuged for one minute and allowed to stand for 10 minutes where the final pH of supernatant was measured by using Hi Indicator pH papers; which has a predominant pH range of 3.5 to 6.0 and 6.5 to 9.0 and categorized accordingly. Scoring criteria for salivary pH was as follows:
Score 0: pH>6.0, Score 1: pH 4.5 – 5.5 and Score 2: pH<4.0
For microbial analysis, the saliva sample was homogenized manually by stirring using a stirrer. Hundred μl of saliva was diluted with 1ml of sterile peptone water to obtain 1:10 dilution of saliva. 100 μl of the diluted saliva was further added to 1 ml of sterile peptone water to obtain a dilution 1:100. This procedure was repeated again to obtain a dilution of 1:1000. This dilution of saliva was used for microbial analysis. The S. mutans was cultured on Mitis Salivarius Kanamycin Bacitracin (MSKB) agar which is the selective culture medium of this organism.
Each litre of MSKB was prepared according to the manufacturer’s instructions by first autoclaving separately 90 gm MS base in 800 ml of deionised water and 200 gm sorbitol sugar in 200 ml deionised water. After combining and cooling to 500 C, 1 ml of 1% potassium tellurite, 2.5 ml of 40 U/ml of bacitracin and 2 ml of 500 μg/ml kanamycin sulphates were added to produce 0.1 U/ml bacitracin and 1 μg/ml kanamycin sulphate. The prepared media was poured into sterile disposable culture plates and refrigerated till inoculation [9]. Using an inoculation loop (2 mm inner diameter) 5 microliter of the 1:1000 dilution samples was streaked on MSKB agar under the strict aseptic conditions. The MSKB agar plates were incubated for 72 hours at 370°C, anaerobically using candle jar. After 72 hours of incubation period, colonies of S. mutans appeared on the culture plates as small, rough, raised and adherent. The colonies so identified were counted using a magnifying glass and the count of S. mutans was expressed as the number of colony forming units/ ml of saliva. As 5 μl of saliva samples were taken for culturing, the number of colonies obtained from this culture was multiplied by 1/5 x 103 and corrected for dilution factor to calculate the number of colonies for 1 ml of saliva [14].
The number of colony forming units was counted with the help of magnifying lens and scored as follows [15] Score 0: negligible, Score 1: <104, Score 2: 104 - 105, and Score 3: >105.
Gram’s staining was performed for morphological identification of gram positive Streptocoocci. Identification of S. mutans was confirmed by biochemical tests using Hi Strep identification kit (Himedia REF KB005A). It is a standardized, colorimetric identification system utilizing twelve conventional biochemical tests. The tests are based on the principle of pH change and substrate utilization. Isolated Streptococcus mutans colony was picked up with sterilised inoculation loop and inoculated in 5 ml Brain Heart Infusion Broth and incubated at 370°C for 4 hours until the inoculum turbidity reaches 0.1 OD at 620 nm. Calibrated syringe was used to carry the broth and inoculated in HiStrep identification strip. On incubation, organisms undergo metabolic changes which are indicated as a colour change in the media that is either visible spontaneously or after addition of a reagent.
For validation of our results, we procured the standard strain of Streptococcus mutans MTCC 890 (Reference number 43392, MTCC, Chandigarh). A colony was picked up with sterilized inoculation loop and inoculated in 5 ml Brain Heart Infusion Broth and incubated at 370 C for 4 hours. Calibrated syringe was used to carry the broth and inoculate in HiStrep identification strip (Himedia KB005A).
Statistical analysis: The collected data was assessed using students t’ and chi-square test. P value <0.05 was considered to be statistically significant (*P<0.05).
There were 7(28%) boys and 18(72%) girls in caries active; 14(56%) boys and 11(44%) girls in caries free group respectively. The Students ‘t-test’ value of DMFT and def was 9.23 and 7.69 respectively. The data for both the study groups was statistically significant with p value at 0.00001 (p<0.05) (Table 1).
Table 1. Gender wise distribution of and comparison of DMFT and def scores in dental caries active and caries free groups.
| Variable | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| GROUPS (N=50) |
DMFT | Def | |||||||
| Mean | SD | t-value | p-value | Mean | SD | t-value | p-value | ||
| Dental caries active (25) | |||||||||
| Boys (7) (28%) |
Girls (18) ( 72% ) |
1.60 | 0.87 | 9.2376 | 0.00001* | 3.12 | 2.03 | 7.6949 | 0.00001* |
| Dental caries free (25) | |||||||||
| Boys (14) (56%) |
Girls(11) (44%) |
0.00 | 0.00 | 0.00 | 0.00 | ||||
*p<0.05
Quantitative Analysis of Salivary Flow Rate and buffering capacity and log Streptococcus mutans CFU count:
Statistically significant difference was observed on comparison of salivary flow rate, buffering capacity and log Streptococcus mutans CFU counts in saliva of dental caries active and caries free group with p value at 0.00001 (p<0.05) (Table 2).
Table 2. Quantitative analysis of salivary flow rate, buffering capacity and log Streptococcus mutans CFU counts in dental caries active and dental caries free groups.
| Variable | Groups (N=50) | |||||||
|---|---|---|---|---|---|---|---|---|
| Dental caries active (25) | Dental caries free (25) | |||||||
| Mean | SD | t-value | p-value | Mean | SD | t-value | p-value | |
*p<0.05
Qualitative Analysis of Salivary Flow Rate and buffering capacity
The subjects with no caries showed significantly high flow rate in comparison to caries active subjects (p<0.05). The salivary flow rate of >0.7 ml/minute was found in 19 (76%) subjects of caries active and 25 (100%) subjects of caries free group respectively. The salivary flow rate of 0.3 – 0.7 ml/minute was found in 6 (24%) subjects of caries active group but was not found in caries free subjects. None of the subjects in both groups had low salivary flow rate of <0.3 ml/minute. The data of both the groups was statistically significant with p value at 0.00903 (p<0.05). In dental caries active and caries free group, the buffering capacity of pH>6.0 was found in 4 (16%) and 24 (96%) subjects respectively. The buffering capacity of pH 4.5 – 5.5 was found in 11(44%) and 1(4%) in dental caries active and caries free group respectively. The buffering capacity of pH<4.0 was found in 10 (40%) subjects of caries active group. None of subjects had buffering capacity of pH 4.0 in caries free group. The data of both the group was statistically significant with p value at 0.00001 (p<0.05) (Table 3).
Table 3. Qualitative analysis of salivary flow rate, buffering capacity and log Streptococcus mutans CFU counts in dental caries active and dental caries free groups
| Groups (N=50) | Variable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Salivary flow rate (ml/min) | Buffering capacity | Log Streptococcus mutans (CFU counts | |||||||
| Score 0 | Score 1 | Score 0 | Score 1 | Score 2 | Score 0 | Score 1 | Score 2 | Score 3 | |
| Dental caries active (25) | 19 | 6 | 4 | 11 | 10 | 0 | 3 | 10 | 12 |
| Dental caries free (25) | 25 | 0 | 24 | 1 | 0 | 2 | 12 | 9 | 2 |
| Chi-square | 6.8181 | 32.6192 | 14.5953 | ||||||
| p-value | 0.00903* | 0.00001* | 0.0022* | ||||||
*p<0.05
Microbiological Analysis
The subjects in caries active group showed significantly high Streptococcus mutans counts in comparison to subjects in caries free group. In dental caries active group, none of the subjects had score 0, while in caries free group there were 2 (8%) subjects who had zero score. In dental caries active and free group, score 1 was found in 3 (11%) subjects and 12 (48%) subjects respectively. Score 2 was found in 10 (40%) subjects in dental caries active and 9 (30%) subjects in dental caries free group respectively. In caries active and caries free group, score 3 was found in 12 (48%) and 2 (8%) subjects respectively. The data of both the groups was statistically significant with p value at 0.0022 (p<0.05) (Table 3) Streptococcus mutans are gram positive cocci appearing as dark blue or violet on Gram’s staining. The individual cocci were spherical or oval in shape, 0.5 – 1.0 μm in diameter and arranged in chains. (Figure 1) Growth of S. mutans on MSKB culture media is seen as bluish, small, rough, raised and adherent colonies (Figures 2 and 3).
Biochemical analysis
I. HiStrep TM identification strip (Himedia KB005A) used for biochemical analysis showed the following results -
II. Voges Proskauer’s test: Positive reaction (Pinkish red)
III. Esculin hydrolysis test: Positive reaction (Black)
IV. PYR: Positive reaction (Cherry red)
V. ONPG: Negative reaction (Colourless)
VI. Arginine utilisation: Positive reaction (Purple/ Dark Purple)
VII. Glucose: Negative reaction (Pinkish red/ Red)
VIII. Lactose: Negative reaction (Red/ Pink)
IX. Arabinose: Positive reaction (Yellow)
X. Sucrose: Positive reaction (Yellow)
XI. Sorbitol: Positive reaction (Yellow)
XII. Mannitol: Positive reaction (Yellow)
XIII. Raffinose Positive reaction (Yellow)
Validation
The biochemical test results of standard strain of Streptococcus mutans MTCC 890 (Reference number 43392, MTCC, Chandigarh) were similar to the results of our study sample.
It is known that good oral health is an integral component of good general health. Although enjoying good oral health includes more than just having healthy teeth, many children have inadequate oral and general health because of active and uncontrolled dental caries. Dental caries is a disease process that afflicts a large proportion of the world’s population. The aetiology and pathogenesis of dental caries is multi factorial. Numerous host, agent and environment factors play a role in the development of dental caries [15,16]. Dental caries is a major public health problem due to high prevalence in all regions of the world. The evaluation of risk of dental caries is important as it gives an opportunity to implement preventive measures in an exposed population [17]. The concept of the prediction of human dental caries risk has existed for many years. The assessment of caries risk may involve predictors such as specific causally related risk factors which may also involve predictors that are associated with caries but not causally related risk factors. Caries risk predictors may be found among the microbiota (dental plaque), the diet (carbohydrates) and the host (teeth). All three are indispensable for caries development. Saliva may be added in view of its potentially powerful influence on the caries process [18]. Among all the factors, saliva is the important factor which influences the development of dental caries as teeth are in constant contact and are bathed by the saliva [16]. Research on saliva has thus become an important field in dentistry and oral biology.
This study attempts to evaluate and compare the relationship between salivary flow rate, buffering capacity and isolation of Streptococcus mutans using Mitis Salivarius Kanamycin Bacitracin (MSKB) medium from caries active and caries free school children aged 9-12 years and to determine the efficacy of MSKB as a selective medium for isolation of Streptococcus mutans.
Under resting conditions without the exogenous stimulation, there is slow flow of saliva which keeps the mouth moist and lubricates the mucous membrane. When the flow is unstimulated, the parotid, submandibular, sublingual and minor mucous glands (MMGs) contribute about 25%, 60%, 7 to 8% and 7 to 8% respectively to whole saliva but when flow is stimulated, the parotid gland contribution increases by at least 10% [19]. Protective properties of saliva which increase on stimulation include salivary clearance, buffering power and degree of saturation with respect to tooth mineral. These benefits are maximized when saliva is stimulated after the consumption of fermentable carbohydrates, by reducing the fall in plaque pH leading to demineralization and by increasing the potential for remineralization [20]. Hence unstimulated saliva is useful to analyse salivary gland status while stimulated saliva is useful for the study of functional reserve [21]. So in our study, paraffin stimulated whole saliva was used as sample.
Several methods of collecting saliva are available such as draining method, spitting method, suction method and swab method. According to Dawes [19], suction method is the most reproducible; hence this method of saliva collection was employed in our study. Measurement of salivary flow rate is used as a screening method in a population to identify people with low salivary flow which is often but not always related to caries susceptibility and activity [22]. In general higher the flow rate, higher the buffer capacity, faster the clearance and lesser is the microbial attack [23].
Tenovuo [22] reported that salivary flow rate is the most important single parameter, while considering possible association with caries activity and is an important threshold limit of an individual. Low salivary flow rate and dental caries prevalence supports the association between salivary flow rate and dental caries [24]. In our study, statistically significant difference in paraffin stimulated salivary flow rate was observed in between caries active and caries free group. The caries free group had significantly higher stimulated salivary flow rate in contrast to caries active group. The results of our study are similar to the results of the studies conducted by, Mass et al. [25], Azevedo et al. [26], Gopinath et al. [27], Al-Zahawi et al. [28], Aminabadi et al. [29], Fiyaz et al. [30] and Srinivasulu et al. [31]. In contrast no significant difference in association between salivary flow rate and dental caries experience has been reported by Preethi et al. [13], Parvinem et al. [32] and Bretz et al. [33].
Oral cavity is quite frequently exposed to components whose pH differs from normal pH (6.5–7.5) of saliva and these components may cause damage to teeth or mucosal surface. Buffering agents in saliva, however try to bring the pH back to the normal range as fast as possible. In resting saliva the major buffering agent is inorganic phosphate and in stimulated saliva it is carbonic acid / bicarbonate system. At very low pH (4-4.5) salivary proteins also display some buffering action [22].
Four most common methods used for estimating the buffering capacity of saliva exist like the Ericsson’s method modified for smaller volumes, Bratthall and Hager’s method, the Dentobuff method proposed by Ericsson and Bratthall in 1989 and the electrometric method [22]. A study conducted by Ericson and Bratthall revealed that all the four methods correlated well. Being simple and less technique sensitive, Ericsson’s method modified for smaller volumes was used in our study to estimate the salivary buffering capacity.
At population level salivary flow rate and buffer capacity correlate positively but among individuals many exceptions exist. Low flow rate combined with low or moderate buffer capacity clearly indicates poor salivary resistance against microbial attack. Among these patients the clearance of micro-organisms is slow and residual saliva spreads as a thin film on oral surfaces, ranging from 0.5-1.0 ml. Fermentable carbohydrates dissolved in this small volume of saliva would not be neutralized rapidly due to the low buffer capacity but the taste of sugar often together with optional flavoring agents, stimulates the salivary glands to respond in a few seconds with an increased salivary flow rate. The volume of saliva will increase until a swallow is initiated and swallowing clears some of the sugar from the oral cavity. A significant positive correlation between caries prevalence and the residual salivary flow after swallowing has been reported and if the buffer capacity is poor then the acidic attack will be prolonged. The oral cavity is frequently exposed to components whose pH differs from saliva's normal pH (6.5-7.5). These components may cause damage to teeth (erosion) or mucosal surfaces. Buffering agents in saliva however, try to bring the pH back to the normal range as fast as possible [22].
In our study we observed a statistically significant difference in the buffering capacity of paraffin stimulated saliva in caries active and caries free groups. The subjects with no caries showed significant high buffering capacity in comparison to subjects with high caries activity. The results of our study are in accordance with studies conducted by Farsi et al. [17], Malekipour et al. [34], Gudkina et al. [35], Sharma A et al. [36], Motamayel FA et al. [37], Kuriakose S et al. [38], Shetty et al. [39] and Bhayat et al. [40]. In all these studies, the dental caries prevalence was significantly higher in caries free than caries active groups. Our results are however in contrast to the results of the study conducted by Preethi BP et al. [13]. The reason for this could be the fact that other associated factors like microflora, diet and retention of food might have dominated the buffering capacity to initiate caries which is a known multi factorial disease.
Streptococcus mutans exhibit putative cariogenic traits such as high potential for acidogenesis and acid tolerance. Primary interest in the Streptococcus mutans as a caries predictors stems from the fact that caries conductive conditions on coronal as well as root surfaces in humans are associated with elevated levels of this organism in saliva and/ or in plaque [41]. Due to the positive numerical association of counts of Streptococcus mutans with dental caries and its linkage with carbohydrate consumption, recently the main focus has shifted to Streptococcus mutans counts in saliva samples. Hence, Streptococcus mutans counts may serve not only as a caries predictor but also as an indicator of carbohydrate consumption which is another caries risk factor.
In our study, Streptococcus mutans were cultured on saliva samples using Mitis Salivarius Kanamycin Bacitracin agar, which is the selective culture medium for the growth of S. mutans.
We observed a statistically significant difference in Streptococcus mutans counts among subjects of caries active and caries free groups. The subjects in caries active group showed significantly high Streptococcus mutans counts in comparison to subjects in caries free group. In dental caries active group, none of subjects had negligible salivary S. mutans count while in caries free group, there were 2 (8%) subjects who had zero salivary S. mutans counts. Our results are in accordance with studies conducted by Hegde PP et al. [5], Hebbal M et al. [6] Gamboa F et al. [8], Okada et al. [42], Hegde et al. [43], Simon et al. [44], Miravet et al. [45], Ge et al. [46], Thaweboon et al. [47], Leal et al. [48] and Pannu et al. [49]. However studies conducted by Farsi et al. [17] and Granath et al. [41] showed no significant relation between dental caries experience and S. mutans. In the study conducted by Granath et al. [41], the authors attribute the reason for the results to a probably skewed distribution subjects in DMFT/def classes for lower bacterium classes than for higher bacterium classes.
A new medium, MSKB composed of Mitis Salivarius agar base, Kanamycin sulphate and Bacitracin, has been developed which is more selective for recovery of Streptococcus mutans. Initial studies showed that traditional media i.e. MSB, TSY20B and GSTB were not selective enough to enable counting of mutans without the aid of a microscope for morphogenic differentiation. The new selective medium, MSKB has been optimized to reduce the growth of non–mutans and thus eliminating false positive results when used in a caries diagnostic test [9,15]. Contradictory findings have been noted by Yen et al. [50] who observed that the selectivity of MSKB and MSB were the best as compared to GSTB and TSY20B but the recovery rate of MSB was better followed by MSKB, GSTB and TSY20B and stated that MSB was the most suitable medium for detection of cariogenic Streptococcus mutans based on its characteristic in differentiation, reliability and recovery rate. Further comparative studies on larger sample size with other variables like diet, type of saliva, oral hygiene habits by using different selective media for growth of Streptococcus mutans from salivary sample in caries active groups are advocated to farther our study.
The mutans selective growth medium, MSKB is an excellent diagnostic medium for growth of Streptococcus mutans. Salivary variables like flow rate and buffering capacity is a valuable diagnostic tool to assess caries experience and predict caries risk.