e-ISSN: 2319-9849
e-ISSN: 2319-9849
1Research Scholar, St. Ann’s Degree for Women, Mehdipatnam, Hyderabad, Telangana, India
2Department of Inorganic and Analytical Chemistry, Andhra University, Visakhapatnam, India.
3Head of the Department of Inorganic and Analytical Chemistry, Andhra University, Visakhapatnam, India
4School of Environmental Science, JNIAS, Hyderabad, India
Received Date: 23/04/2021; Accepted Date: 21/07/2021; Published Date: 30/07/2021
Visit for more related articles at Research & Reviews: Journal of Chemistry
Due to its efficient and low-cost activity, calcium carbonate, a constituent of chalk powder is considered to be one of the strongest adsorbents for the treatment of groundwater that holds inorganic metals. Based on the calcium content, surface area and morphology, chalk powder is used to achieve a high potential rate of arsenic adsorption. The adsorption process is also aided by the porosity and chemical composition of chalk powder. At different settings, the rate of arsenic adsorption is found to be directly proportional to parameters such as contact time, adsorbent dosage, concentration and pH. At different temperatures, the Freundlich adsorption isotherm ruled equilibrium adsorption with the best correlation coefficients (0.999).
At various concentrations, the equilibrium adsorption potential was demonstrated by pseudo second - kinetic order. Negative values of Gibbs energy, enthalpy and entropy indicated that the adsorption mechanism is instinctive and endothermic, with no randomness at the solute-liquid interface. The separation factor RL was found to be in the range of 0< R< 1, indicating that Arsenic adsorption onto Chalk powder was excellent. The above findings led to the decision to use chalk powder as a low-cost adsorbent for removing arsenic from an aqueous solution.
Arsenic adsorption, Freundlich isotherm, Gibbs energy, Enthalpy, Entropy, Calcium carbonate (Chalk Powder).
For the research's foundation, the uniqueness of the adsorbents is taken into account. Adsorption potential, surface area, porosity, critical functional classes, cost and desorption likelihood are all different for each adsorbent. For arsenic adsorptive tests, chalk powder has been regarded as the most powerful adsorbent. Calcium carbonate, with small quantities of silt and clay, makes up chalk powder. The porous calcium carbonate is the most versatile material, with applications in a variety of industries. According to research findings, there are only a few references for the removal of heavy metals using chalk powder. The sorption capability of chalk powder containing clay minerals was found to be increased [1]. With CaCO3 study, chalk powder contributed to the adsorption capability [2-4]. Because of the larger inner spaces in its structure, Ca2+ ions are replaced by arsenic metal ions as a result of adsorption at a larger space, resulting in high arsenic removal efficiency [5].
Arsenic is primarily present in groundwater at concentrations of up to 10 parts per billion (ppb), where it exists as waterinsoluble arsenic anions or as arsenic molecules. In some areas of Bangladesh, groundwater concentrations may exceed 100 parts per billion. According to previous studies, Hyderabad's groundwater contains 10-150 μg/L of arsenic, which can be removed by water purification methods such as adsorption, coagulation, ion exchange and membrane filtration. As (V) is easier to strip than As (III) and it can be pre-oxidized to As (V) for convenience. The adsorption method is used in this study to remove As (III) by using chalk powder.
a. To develop a control technology for removing arsenic from groundwater.
b. Assess the adsorbent's adsorption potential and strength and adjust the adsorbent to improve its efficacy.
c. To develop a low-cost, environmentally sustainable method for removing arsenic from water using low-cost materials.
Adsorbent Selection Methods and Materials
The physical properties of chalk powder rule the adsorption phase, which is physical adsorption due to the decomposition aspect, when calcium carbonate is heated to 900oC. The chalk powder has achieved the best eradication of arsenic from groundwater and confirmed 89% arsenic elimination in a screening analysis due to the porous morphology and texture of the surface with a pleasant crystallized structure of CaCO3 and the inclusion of other ions such as silica, iron with other small particles and their reactivity. Chalk powder was selected as an adsorbent for the removal of arsenic from an aqueous solution based on these considerations.
The Function of Arsenic and Chalk Powder Contact Time
The adsorption method for removing metal efficiently in order to obtain potable water for our survival is aided by contact time. Using 1.0 g of chalk powder and a 50 μg/L arsenic concentration, the effect of contact time on arsenic adsorption was investigated using contact times of 10, 20, 30, 40 and 50 minutes. A slow approach to equilibrium was observed during the preliminary stage. Furthermore, the maximum arsenic removal was estimated at 20 minutes (optimum contact time), which was due to adsorbent properties that could influence the time needed to achieve equilibrium.
The versatility of Arsenic ions was good due to the dilution in lower concentrations and they drove the entire region of solvent media according to Debye-Huckel theory, as shown in Figure 1. Chromium adsorption onto Chalk powder [6] yielded similar results. Calcium arsenate precipitation (Ca3 (AsO4)2) is formed when Arsenic and chalk powder come into contact for a long time. Since this precipitation is unstable, the rate of Arsenic removal drops as the adsorption period increases (2013).
Dosage's Importance in Arsenic Adsorption
The optimal dosage has a direct impact on the amount of adsorbed adsorbate. Figure 2 shows that 2 gm of adsorbent removed 89% of arsenic after 20 minutes of optimum contact time at ambient temperatures ranging from 25°C to 27°C. The extra dose i.e., more than 2 gm provides a greater surface with more binding sites for the metal ion but the adsorption process rate has slowed down to the overlapping of active sites at higher dosages. Since there is no increase after 2 gm due to the conglomeration of exchanger particles, 2 gm was chosen as the optimal dose for further research. The same results were observed during aniline adsorption on graphene oxide [7].
Chalk Powder's Ability to Expel Arsenic at Varying Concentrations
Figure 3 depicts the removal capabilities of Arsenic (III) with various Arsenic initial concentrations of calcium-bearing adsorbent (Chalk powder). Figure 3 shows that the adsorbent had the best Arsenic removal potential at a concentration 42.6 μg/L with 90% arsenic removal. Since co-anions do not interact with the adsorption mechanism, the degree of arsenic removal was higher at higher concentrations.
The particle size, porosity, shape and precise surface area of the adsorbent all play a role in arsenic adsorption by chalk powder. The formation of insoluble calcium arsenate salt is triggered by this process, which involves arsenate anions and calcium ions [8]. Due to the hindrance of calcium arsenate, the rate of adsorption decreases as arsenic concentrations rise.
The Effect of PH on Arsenic Removal
The sorption ability of arsenic is influenced by the pH parameter, which is dependent on the surface charge and ionisation rate of the adsorbent surface [9]. For the unit of adsorbent shown in Figure 4, the capacity to remove arsenic intensified from pH 2.0 to 11.0. At low pH, arsenic ions compete with H+ ions for binding on the chalk particle surface, while at high pH, the negatively charged adsorbent surfaces were accessible for metal ions to bind [10] consequently, the optimal pH ranged from 9.8 to 11.0. Furthermore, precipitation of Ca3(AsO4)2 can obstruct the adsorption process, slowing it down. Removal of Cr (III) and Ni (II) from tannery effluent using calcium carbonate coated bacterial magnetosomes [11] produced identical results.
Arsenic Adsorption at Various Temperatures
Figure 5 shows that 72% - 95% arsenic removal efficiency was observed between 40°C and 60°C temperatures with initial arsenic concentrations of 14 μg, 28.6 μg and 42.6 μg using defined parameters of 20 min contact time, 2 gm dose and 11.0 pH. According to the current studies, the sorption capacity advanced at high temperature with concentrations of 14 μg/L and 42.6 μg/L and the sorption rate is vice versa with a concentration of 28.6 μg/L, noting that the adsorption phenomenon is temperature–independent, implying that the process can be operated at room temperature [12] i.e., with no additional energy.
The low adsorption potential at 28.6 μg/L concentration could be attributed to CaCO3 super saturation at higher temperatures, which could result in scales forming on the surface as a result of precipitation [13]. This could reduce the adsorption efficiency by inhibiting arsenic uptake. However, scaling may occur due to excessive solubility in the bulk, which is not always related to temperature. The fact that the adsorption process is endothermic is known.
The interpretation of adsorption isotherms and their equilibrium onto adsorbents has been the subject of many studies [14,15]. Linear regressions scrutiny [16] defined the distribution of adsorbates, the analysis of adsorption isotherm models and their assumptions. As a result, linear isotherm modelling has seen a lot of use.
The Langmuir Adsorption Isotherm
The Langmuir adsorption isotherm model is a tool for calculating the adsorptive ability of various adsorbents [17] and visualising monolayer adsorption on the adsorbent surface. The broad surface area and magnitude of the pore are given by the adsorbent's porosity. This model interprets surface coverage by balancing the relative amounts of adsorption and desorption: adsorption is proportional to the fraction of accessible adsorbent surface and desorption is proportional to the amount of adsorbent surface covered [18]. The Langmuir linear equation [19] is used in our investigation to achieve these principles.
Figure 6 shows that the Langmuir adsorption isotherm was unable to determine the dimension of adsorption, as well as the magnitude of porosity and surface area. The slope (-ve values) and intercept of the plot described in Table 1 were measured using the lines that emerged from the axis. At lower arsenic concentrations than the Freundlich and Temkin isotherms, the correlation coefficients (R2) ranged from 0.394 to 0.739. As a result, the Langmuir adsorption isotherm model does not match the arsenic adsorption onto chalk strength.
| S. No | Parameters | Results |
|---|---|---|
| 1. | Surface area | 10.5 cm2 |
| 2. | Particle size | 236 µm |
| 3. | Particle density | 0.27 |
| 4. | Moisture content | 11% |
| 5. | Porosity & void ratio | 59 |
| 6. | Apparent density | 0.25 |
| 7. | Real density | 0.78 |
Table 1. Physical properties of chalk powder.
Isotherm of Freundlich Adsorption
The surface heterogeneity, scattering of active sites and their energies are all defined by the Freundlich isotherm [20]. As a result, adsorption experiments on heterogeneous surfaces were held out using this isotherm in linear form [21,22] which generated the best equilibrium results with strong correlation coefficients [4]. Figure 7 shows a linear shape derived from the plot of log qe versus log Ce. Table 2 shows outstanding R2 values that are higher than the Langmuir and Temkin isotherms, ranging from 0.971- 0.999. It is clear that the superior removal of arsenic by chalk powder corresponded to the Freundlich isotherm in most cases. Adsorptive removal of Chromium from synthetic wastewater into chalk powder [22] yielded the same results.
Temkin Adsorption Isotherm
The Temkin isotherm assumes that the decrease in sorption heat contributed a linear form, as described by the Freundlich equation [23]. Because of sorbate/sorbent interactions, the heat of sorption of all the molecules in the layer will decrease linearly [4,8]. According to Figure 8, the linear association is marginally visible on the adsorptive analysis of arsenic with chalk powder when the Temkin isotherm model assumptions are moderately followed. Since R2 values (0.987-0.993) from Table 2 provide better correlation coefficients, the Temkin adsorption isotherm could also describe the arsenic adsorption process onto chalk powder.
| S. No | Parameters | Langmuir Adsorption Isotherm | ||||
|---|---|---|---|---|---|---|
| Temperature °C | ||||||
| 0°C | 20°C | 40°C | 60°C | 80°C | ||
| 1. | R2 | 0.394 | 0.968 | 0.999 | 0.976 | 0.739 |
| ASS | 0.567 | 0.113 | 0.007 | 0.099 | 0.341 | |
| Q0 | -0.001 | -0.002 | -0.002 | -0.002 | -0.002 | |
| bL | 0.367 | 0.416 | 0.439 | 0.489 | 0.489 | |
| Freundlich Adsorption Isotherm | ||||||
| 2. | R2 | 0.971 | 0.999 | 0.996 | 0.999 | 0.995 |
| ASS | 0.107 | 0.006 | 0.022 | 0.035 | 0.060 | |
| Log kf | 0.835 | 0.841 | 0.715 | 0.846 | 0.906 | |
| l/n | -0.027 | -0.224 | -0.012 | -0.224 | -0.231 | |
| Temkin Adsorption Isotherm | ||||||
| 3. | R2 | 0.989 | 0.993 | 0.993 | 0.990 | 0.987 |
| ASS | 0.005 | 0.003 | 0.003 | 0.004 | 0.006 | |
| aT | 0.294 | 0.327 | 0.352 | 0.377 | 0.401 | |
| bT | 0.327 | 0.430 | 0.440 | 0.541 | 0.642 | |
Table 2. Adsorption isotherm constants and their values of statistical comparison.
Adsorption kinetics analysis is crucial in determining the rate and time needed for solute adsorption in the adsorption phase, mass transfer and chemical reaction. Using kinetic models, the kinetic evaluation of arsenic adsorption was carried out at different time intervals in this analysis.
Pseudo-First-Order Kinetics
The pseudo-first-order kinetic model of Lagergren elucidates the relative rate of occupied and unoccupied adsorbed sites. The R2 values tabulated (0.899) in Table 3, which give the rate of sorption and time needed for arsenic adsorption, were calculated using the linear form of Lagergren equation [10]. However, in this analysis, the low R2 data suggest that the Figure 9 had weak correlation and a nonlinear structure. As a result of these findings, it was stated that the Arsenic adsorptive experiments on chalk powder do not obey the pseudo first-order kinetics. Adsorption tests of Cr (III) and Ni (II) from tannery effluent using calcium carbonate coated bacterial magnetosomes [24] revealed a similar impression.
| S. No | Parameters | Arsenic Concentration (10 µg/L) |
Arsenic Concentration (20 µg/L) |
Arsenic Concentration (30 µg/L |
Arsenic Concentration (40 µg/L) |
Arsenic concentration (50 µg/L) |
|---|---|---|---|---|---|---|
| Pseudo First Order Kinetic Model | ||||||
| 1. | R2 | 0.899 | 0.931 | 0.875 | 0.750 | 0.839 |
| ASS | 0.014 | 0.007 | 0.019 | 0.057 | 0.028 | |
| K1 | 0.005 | 0.002 | 0.003 | 0.002 | 0.002 | |
| Pseudo Second Order Kinetic Model | ||||||
| 2. | R2 | 0.950 | 0.950 | 0.954 | 0.951 | 0.807 |
| ASS | 0.004 | 0.004 | 0.004 | 0.046 | 0.038 | |
| K2 | 0.290 | 0.403 | 0.658 | 1.001 | 0.612 | |
| Elovich Model | ||||||
| 3. | R2 | 0.909 | 0.951 | 0.966 | 0.931 | 0.943 |
| ASS | 0.006 | 0.004 | 0.002 | 0.007 | 0.005 | |
| a | -8.571 | -4.319 | -4.898 | -5.949 | -4.529 | |
| b | 18.66 | 10.23 | 10.03 | 10.96 | 9.816 | |
| Intraparticle Diffusion Model | ||||||
| 4. | R2 | 0.948 | 0.944 | 0.949 | 0.870 | 0.969 |
| ASS | 0.005 | 0.005 | 0.004 | 0.020 | 0.002 | |
| kid | -1.548 | -0.772 | -0.873 | -1.036 | -0.066 | |
| I | 14.70 | 8.204 | 7.718 | 8.012 | 3.109 | |
Table 3. Kinetic parameters for adsorption of Arsenic by Chalk powder.
Pseudo-Second-Order Kinetic Model
The benefit of the pseudo-second kinetic model is that K2 can be used to measure the equilibrium adsorption potential. The rate of adsorption is determined by adsorption capability rather than adsorbate concentration [25]. The plot of t/qt versus t, as shown in Figure 10, generated a linear plot. The K2 and R2 values obtained from this graph were calculated using the pseudosecond- order kinetic model shown in Table 3, which provided the strongest correlation between the experimental and theoretical results. As a result, the potency of arsenic adsorption onto chalk powder matched the pseudo-second-order kinetic model very well.
Implementation of the Elovich Model
Zeldowitsch's Elovich kinetic model predicts mass and surface diffusion, as well as the relationship between the rate of adsorption and the amount of solute adsorbed [22]. As shown in Table 3, the rates of adsorption (α) and desorption (β) from slope and intercept were determined using negative and positive values, respectively.
Since the rate of adsorption is indirectly proportional to the amount of solute adsorbed, the graph (Figure 11) of qt contrasting ln(t) determined that adsorption on the heterogeneous surface of the adsorbent is physical adsorption. According to the results of this analysis, the R2 values (0.909) in Table 3 were not linearly correlated.
Model of Intra-Particle Diffusion
Mass transfer (film diffusion), surface diffusion, pore diffusion and adsorption of solute onto active sites [15] are the four mechanisms involved in intra particle diffusion. In this study, the intercepted values of I (boundary layer thickness) were reduced, resulting in a reduced effect of the boundary layer, resulting in the nonlinear function shown in Figure 12. In comparison to other models in Table 3, correlate efficient values are lower (0.870), indicating that mass transfer has become a rate-controlling stage. As a result, the arsenic adsorption mechanism was not regulated by the intra particle diffusion model (Figures 13 and 14).
The changes in Gibbs energy, standard enthalpy and entropy for each temperature are computed using the Van't Hoff equation and the plot with equilibrium constants.
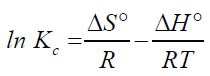
Table 4 shows the results of thermodynamic tests. The slope and intercept of the plot of ln qe/ce versus qe give the values of ΔHo and ΔSo (0.258 and -88.75) shown in Table 5. In the temperature range of 273 – 353 K, ΔGo was measured and negative results were recorded, inferring the feasibility and spontaneous nature of the adsorption reaction [6] the positive value of Enthalpy indicates an endothermic reaction, while the negative value confirms that there is no randomness at the solid-solution interface during arsenic adsorption.
| S. No | Temperature | DG (KJ/mol) | DS (KJ/mol) | DH (KJ/mol) |
|---|---|---|---|---|
| 1. | 273 | -17.02 | -88.75 | 0.258 |
| 2. | 293 | -21.68 | ||
| 3. | 313 | -19.25 | ||
| 4. | 333 | -23.80 | ||
| 5. | 353 | -28.46 |
Table 4. Thermodynamic parameters of Arsenic adsorption by Chalk powder.
| S. No | Temperature (°C) | Concentration of Arsenic (µg/L) and RL values | ||
|---|---|---|---|---|
| 10 µg/L | 30 µg/L | 50 µg/L | ||
| 1. | 0 | 0.1001 | 0.033 | 0.200 |
| 2. | 20 | 0.1002 | 0.033 | 0.200 |
| 3. | 40 | 0.1002 | 0.033 | 0.200 |
| 4. | 60 | 0.1002 | 0.033 | 0.200 |
| 5. | 80 | 0.1002 | 0.033 | 0.200 |
Table 5. Equilibrium parameter RL values at different concentrations and temperatures.
Equilibrium Criteria
The tests of arsenic adsorption onto chalk powder RL at various temperatures were reported as RL< 1 (0.1), indicating that the arsenic adsorption is a favourable process.
The outstanding adsorption ability for Arsenic metal was attributed to the physical and chemical properties of Chalk powder. The percentage of arsenic removal onto chalk powder from its solution was 85%, confirming that it is the qualified adsorbent for arsenic adsorption from aqueous solutions, according to the survey of parameters such as contact time, adsorbent dose, pH and initial metal ion concentration. The analysis of arsenic adsorption on chalk powder revealed the Freundlich adsorption isotherm and its effective correlation coefficients. The kinetic parameters showed that the current study is a good match to the pseudosecond kinetic order and the thermodynamic constants showed that the process is endothermic and random and that the RL value is favourable. As a result of these findings, we can infer that chalk powder can play an important role in low-cost, environmentally, friendly technology because it has a higher adsorption strength and potential for Arsenic removal.
I am in gratitude to my guide Dr. B.B.V. Sailaja, Head of the Department of Inorganic and Analytical Chemistry at Andhra University and my co-guide Dr. D. Sirisha David, whose passion, experience and meticulous attention to detail have been an inspiration and kept my work on track since my first encounter with this paper and would not have been possible without their help. I'm also greatful to St. Ann’s Degree College for Women in Mehdipatnam, Hyderabad, India, for allowing me to complete my lab project in the college's research lab.