ISSN: 2321-6204
ISSN: 2321-6204
Department of Biochemistry, Kebbi State University of Science and Technology, Aliero, P.M.B. 1144, Birnin Kebbi, Kebbi State, Nigeria
Received date: 20 May 2014 Accepted date: 22 June 2014
Visit for more related articles at Research & Reviews: Journal of Food and Dairy Technology
There are more than 400 known species of edible insects. Many species of these insects have been used as traditional foods among various communities. Insects have played an important part in the history of human nutrition in Africa. Thus the aim of this research is to evaluate the nutritional value and mineral composition of edible Zonocerus variegatus (grasshopper). To evaluate the nutritional value, proximate, antinutrient and mineral elemental composition were analyzed using standard methods. The result of the proximate analysis revealed the following: Moisture (5.33±1.16%), Ash (11.50±3.04%), Crude lipid (49.33± 2.08%), Crude fiber (2.03±1.01%), Protein (2.19±0.87%) and Carbohydrate content (29.61±4.21%). The concentrations (mg/100g) of the antinutrients in this edible insect are; 11.25±0.46 for oxalate, 2.18±0.24 for phytate and 3.90 ±0.1 for tannin. The mineral composition (mg/kg) revealed, Na, K, Ca, Mg, Fe, Cu and Zn with 0.02±0.00, 0.04± 0.01, 1.12± 0.07, 0.57±0.08, 1.84±0.05, 0.20±0.01 and 1.63±0.01 respectively. This research shows that the edible grasshopper contains significant amount of nutritional and mineral components, with less concentrations of antinutrients. Therefore, it can be recommended especially for children, pregnant women and aged people.
Antinutritional Factors, Grasshopper, Nutrition, Proximate Analysis, Zonocerus variegatus
The deficiency of food resources has become an important issue for modern civilization and most of the developing countries are facing difficulties in providing sufficient food for their population, and thus insufficient intake of protein sometimes cause protein-energy malnutrition [1]. On behalf of mankind, it is being very important to explore and exploit new nonconventional, natural and sustainable food resources.
The search for alternative source of food nutrient remains a perpetual event as human population growth is dynamic and ever increasing under – exploitation and under-utilization of abundant alternative natural resources have now been recognized as one of the militating factors against nutrient glut as intended by the ‘creator’. According to [2], insects have played an important role in the history of human nutrition. Furthermore, insects naturally produce a good biomass as they are an exceptionally productive group in the animal kingdom, constituting about 76% of known species of surviving animals [3], and have one about trillion kg of gross weight in the world.
The consumption of selected insects in diverse forms is a positive response to this imperative. Insect and meat play the same role in the human body. As food, some insects are regular in the village but meat as a stranger [4]. Most people in tropical Africa collect insects for food. The habit is especially well developed among the cultivators of the forest region. It is uncertain whether these insects are eaten because of their nutritional qualities.
A number of insect or their products were used as food in some parts of Nigeria and to a large extent eaten as tidbits or exclusively by children [5]. Ordinarily, insects are not used as emergency food during shortages, but are included as a planned part of the diet throughout the year or when seasonally available [6]. However, as the insects are only seasonally available, preservation by sun-drying is often practiced. Whether or not insects are eaten depends not only on taste and nutritional value, but also on customs, ethnic preferences or prohibitions [7].
The grasshopper, Zonocerus variegatus (Linn.) has a large dry season population in South-western and North-western parts of Nigeria as well as in other regions of Africa [8]. To manage the insects in the interest of food security, they should be made better available throughout the year by inventing organized and controlled production of the insects as mini-livestock and improving the conservation methods [9].
But nutrient analysis and compositions of many grasshopper species is yet to be evaluated. Such data would be much helpful for food consumption studies, in updating food composition tables and in diet therapy. The purpose of the present work is to determine nutritional value and mineral composition, including anti nutrient content of grasshopper.
Sample Collection and Preparation
The dried grasshopper was purchased from market, in Argungu Local Government Area. Kebbi State, Nigeria. It was then taken to the Department of Biological Sciences, Kebbi State University of Science and Technology, Aliero, Nigeria for authentication.
The wings were removed and discarded then ground to powder using mortar and pestle. It was then used for analyses.
Proximate Analysis
Analyses of the samples for moisture, ash and crude fiber were carried out in triplicate using the methods described by [10]. The nitrogen was determined by the micro kjedahl method and the nitrogen content was converted to protein by multiplying with a factor of 6.25. Carbohydrate was determined by the difference.
Determination of Moisture Content
A crucible was weighed as (W1). 5g of the sample was added into the crucible and weighed as (W2), it was then dried in a hot air oven at 8hr per day for 5 days, at 1050C and then cooled in a desiccator and the weight taken as (W3). The dried sample was then returned into the oven for 24hr and readings were taken using the same timing until constant values were obtained to ensure complete drying.
The percentage is obtained by the formula:
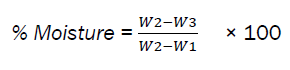
Determination of Ash Content
The crucible was weighed as (W1). 2g of the sample was added into the crucible and weighed as (W2) and then placed in a muffle furnace at 500 0C for 6hr. It was then cooled in a desiccator and the weight was measured again as (W3).
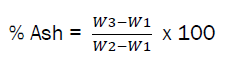
Determination of Crude Fiber
2g of defatted sample was weighed into 1 liter conical flask as (W1). 200ml of boiling 1.25% H2SO4 and 200ml of water were added gently and boiled for 30mins using cooling finger to maintain a constant volume. A muslin cloth was used to filter and distilled water was used in rinsing. Spatula was used to scrape the material back into the flask. 200ml of boiling 1.25% NaOH was added and boiled for 30mins using a cooling finger to maintained a constant temperature. A poplin cloth was used in filtering; the residue was rinsed thoroughly with hot distilled water, and was also rinsed once with 10% HCl.
It was then allowed to dry overnight in the oven at 1050C, then cooled in a desiccator and weighed as (W2), it was then kept in the muffle furnace to ash at 5500C for 90mins. There after it was cooled and weighed as (W3).
Percentage fiber was obtained by the formula:
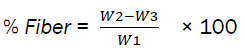
Determination of Crude Fat
A 250ml extraction flask was washed and dried in an oven at 1050C, and the extraction flask was weighed. 30g of the ground sample was weighed into a labeled porous thimble. The thimble mouth was covered with white clean cotton wool. 200ml of petroleum ether was added into 250ml extraction flask. The covered porous thimble was placed into the condenser and the apparatus was assembled for extraction, which continues for 6hrs. The porous thimble was removed and the extraction flask was placed on the water bath to make it free from petroleum ether. Then the weight was taken as (W3).
Percentage fat was calculated as follows:
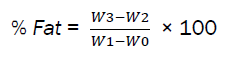
W0 = Weight of empty porous thimble, W1 = Weight of thimble + Ground Sample, W1 - W0 = Weight of ground Sample, W2 = Weight of empty extraction flask, W3 = Weight of extraction flask + oil
Determination of Protein Content
0.5g of sample was weighed into a dried 500ml Szmacro-Kjeldahl flask then 20 ml of distilled water was added, the flask was swirled for few minutes and allowed to stand for 30 minutes. 2 tablets of mercury catalyst were added together with 30ml of conc. H2SO4 using a measuring cylinder. The flask was cautiously heated with low heat on the digestion stand until a clear digest was obtained and was further boiled for 5 hours. The flask was allowed to cool, and then 100ml of distilled water was slowly added to the flask. 10 ml of the digest was transferred into another clean macro kjedahl flask (750ml). The residue was then washed with 50ml distilled water four times and aliquot transferred into the flask. 20ml of H3BO3 indicator solution was added into a 250ml Erlenmeyer flask which was then placed under the distillation apparatus. The 750ml kjedahl flask was attached to the distillation apparatus. 150ml of 10N NaOH was poured through and allowed to cool (below 30 0C), by allowing sufficient cold water to flow through and also regulating heat to minimize frothing and prevent suck back. 40ml of the distillate was collected and then distillation stopped. The NH4-H in the distillate was determined by titrating with 0.01N standard HCl using a 25ml burette graduated at 0.1ml intervals.
The percentage Nitrogen was calculated by the formula below;
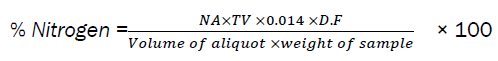
Where; NA = Normality of acid (0.01N), TV = Titer value, DF = Dilution Factor, Volume of aliquot = 10ml
Analysis of Anti-Nutritional Factors
Determination of Phytate Content
4.0g of the sample was soaked in 100ml of 2% HCl for 5 hours and then filtered. 25mls of the filtrate was taken into the conical flask and 5.0ml of 0.3% NH4SCN solution was titrated with a standard solution of FeCl2 containing 0.00195g Fe/ml until a brownish yellow color persisted for 5minutes. 1ml = 1.10mg Phytin-Phorsphorus. The phytate content was calculated by multiplying the value of Phytin-Phorsphorus by 3.55.
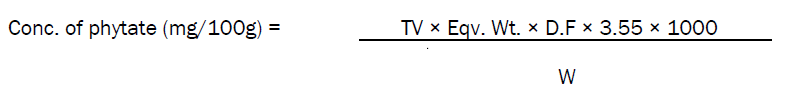
TV = titre value, Eqv. Wt = equivalent weight, D.F = dilution factor, 1000 = conversion factor to mg/100g of sample and W = weight of sample
Determination of Oxalate Content
2.5g of the sample was extracted with 100ml of 2% HCl, 5ml of conc. NH3 and precipitated with CaCl3 as calcium oxalate. The precipitate was then washed with 20ml of 25% H2SO4 and dissolved in hot water, then titrated with 0.05N KMnO4 to determine the conc. of oxalate until a pink end point was observed (1ml of 0.05N= 0.045g oxalic acid)
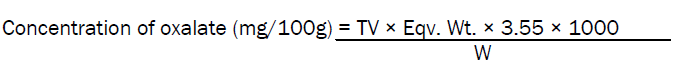
TV = Titre Value, Eqv. Wt = Equivalent Weight, D.F = Dilution Factor, 3.55 = Phytin-Phorsphorus Factor, 1000 = Conversion Factor to mg/100g of sample and W = Weight of Sample
Determination of Tannin Content
0.5g of the sample was weighed into a 10g plastic bottle. 100ml of distilled water was added and shaken for 1hr in a mechanical shaker. This was filtered into 10ml volumetric flask and made up to the mark then 1ml of the filtrate was pipetted into test-tube and mixed with 0.4ml of 0.1M FeCl2 in 0.1N HCl and 0.008M potassium ferrocyanide. The absorbance was measured at 550nm within 10 minutes.
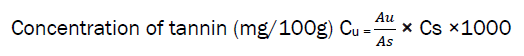
AU = absorbance of unknown sample, AS = absorbance of standard, CS = concentration of standard, CU = concentration of unknown sample and 1000 = conversion factor to mg/100g.
Analyses of Some Mineral Composition
1g of sample was weighed in a crucible, burned on hot plate until the smoke subsides completely and then ashed in a muffle furnace at 500oC for 6 hours. The crucible was transferred into a desiccator and allowed to cool. The ashed sample was dissolved in 1ml of concentrated nitric acid. The dissolved ashed sample was evaporated to dryness on a hot plate. 5ml of 5M hydrochloric acid was added and transferred to 100ml standard volumetric flask. It was then made up to mark with distilled water and filtered. The prepared sample was analyzed for the mineral elements using Atomic Absorption Spectrophotometry.
The results of the proximate analysis are presented in Table 1, while those of anti-nutritional factors and mineral composition are presented in Table 2 and 3 respectively.
Proximate Composition
The results obtained in this research revealed the nutritional contents of the dried edible grasshopper (Zonocerus variegatus). Moisture content was in line with earlier reported results [9, 11, 12], but higher than that obtained by [6]. High moisture content leads to the risk of microbial deterioration and spoilage of food substances.
The result of the ash content was higher than the values reported by [6, 9]. But similar to the work reported by [12]. Ash content of a sample could assume its Mineral content [13].
According to [9] report, fat content was high in the grasshoppers they analyzed which is similar to what was obtained in this research. This indicates that the analyzed grasshopper is rich in fat, which is higher than other grasshopper species reported by [11, 12]. World Health Organization (W.H.O) standard for fat content of edible insects as reported in development of regional standard for edible crickets was 3.3g. Fats are essential in daily human diets as they increase the palatability of foods by absorbing and retaining their flavours [13]. These are also vital in the structural and biological functioning of the cells and help in the transport of nutritionally essential fat-soluble vitamins.
The crude fiber content was comparable with the result obtained by [6, 9], but varied with that of [11, 12]. Variation in crude fiber for different species of grasshopper might be due to different exoskeletons and structure.
Protein content in this research was comparable to the result obtained by [6], but low when compared with the report of [9, 12]. Insect protein could contribute daily protein requirement of human as recommended by National Research Council [14].
The result of the carbohydrate content was higher than the values reported by [12]. World Health Organization (W.H.O) standard of carbohydrate content of edible insects as reported in development of regional standard for edible crickets was 2.2g. Carbohydrate content could be assumed due to the chitinous nature of the grasshopper.
Anti-Nutrient Composition
In this study, tannin was observed to be low compared to the result obtained by [12]. Oxalate upon comparism with that obtained by [9, 12] was observed to be high, while phytic acid is higher compared to that obtained by [12]. These might be due to different species used and hence Zonocerus variegatus is better for consumption than other grasshopper species, as it contains less antinutritional factors.
Mineral Elements Composition
The mineral composition of Zonocerous variegatus showed that calcium, iron and zinc are more abundant compared to magnesium and copper. Values of copper sodium and potassium are moderate, while manganese and lead were not detected. Copper, sodium and potassium were low when compared to the result obtained by [9] but the values of calcium, magnesium, iron and zinc were higher. This shows that grasshopper can be recommended for children, pregnant women, aged people and hypertensive patients.
Even though people consume this grasshopper (Zonocerus variegatus) without knowing its nutritional value, this research shows that the grasshopper has significant amount of nutritional and mineral components. Therefore it can be recommended especially for children, pregnant women, and hypertensive patients.