ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Priya Vijayvergiya1, Shweta Saxena2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Adsorption of Chromium on dye contaminated soil has been studied using batch adsorption technique. This study is carried out to examine adsorption capacity of Chromium on contaminated soil. The influence of contact time, pH, variation of amount of soil, initial Cr Concentration, and particle size also studied. Results revealed that adsorption rate initially increases and equilibrium reached about one hour. Further increase in contact time did not show significant change in equilibrium concentration. The Langmuir and Freundlich isotherm were used to describe the adsorption equilibrium studies. Langmuir isotherm is best fits.
Keywords |
| contaminated soil, chromium, batch method, adsorption, pH, contact time, isotherm. |
I. INTRODUCTION |
| Demands of clothing and apparel increase with the improving sense of fashion and lifestyle; thus textiles are manufactured to meet the growing demand. Heavy metals, particularly, Pb, Cr, Cd, and Cu are widely used for the production of color pigments of textile dyes. These heavy metals which have transferred to the environment are highly toxic and can bio accumulate in the human body, aquatic life and natural water bodies and also possibly get trapped in the soil [1, 2]. Hexavalent Chromium, Cr (VI) is reported to be a powerful carcinogen capable of modifying the deoxyribonucleic acid (DNA) transcription process in both animals and humans that may result in important chromosome aberrations. It is, therefore, essential to remove Cr (VI) from wastewaters of electroplating, dye, cement, leather tanning and paint industries which may contain up to hundreds of mg/L of chromium [3, 4]. Its concentrations in industrial wastewaters range from 0.5 to 270.000 mg·l-1. The tolerance limit for Cr (VI) for discharge into inland surface waters is 0.1 mg·l-1 and in potable water is 0.05 mg·l-1 [5]. In order to comply with this limit, it is essential that industries treat their effluents to reduce the Cr (VI) to acceptable levels. [6]. In Bhilwara, there are many Textil industries and according to study based on NGCM programme in Rajasthan certain areas of Bhilwara having Cr upto 160 to 190 ppm [7]. Chromium is present in the environment in the form of Cr(III) and Cr(VI). These two forms show different chemical, physico-chemical and biochemical properties. Cr (VI) species are more soluble, mobile and bioavailable than Cr (III) species. The presence of these two forms and their relative ratio is dependent on chemical and photochemical redox transformation, precipitation/dissolution and adsorption/desorption reactions [8]. Cr(VI) is known to be a strong oxidant and to be highly toxic. Besides Cr(III) is an essential element for humans, Cr(VI) is highly irritating and toxic to humans and animals [9, 10]. Among the methods used to remove heavy metals from the environment, sorption and biosorption are studied in literature intensively in last decades [11]. The objective of this study was to investigate the Cr (VI) sorption and migration onto a natural soil and the maximum amount of Cr(VI) that can be loaded on this soil [12]. |
II. MATERIAL & METHOD |
| The Batch test were carried out in 250 ml flask using a natural soil as a sorbent. The soil samples were collected from the process house, IV phase, RIICO industrial area, BHILWARA. The soil samples were dried in air for about three weeks. After drying the soil was sieved in order to obtain different particle size distribution (100-300 BSS). Chromium samples were prepared by dissolving a known quantity of potassium dichromate (K2Cr2O7) in double-distilled water and used as a stock solution and diluted to the required initial concentration. A 2g natural soil was mixed with 100ml of the aqueous solutions of various initial concentration (0.5mg/L, 1mg/L, 2mg/L, 3mg/L and 4 mg/L) of chromium (VI) in each flask. The stirring speed was kept constant at 120 rpm. The flasks were shaken at a constant rate, allowing sufficient time for adsorption equilibrium. It was assumed that the applied shaking speed allows all the surface area to come in contact with heavy metal ions over the course of the experiments. The study was performed at room temperature to be representative of environmentally relevant condition. The pH of the solution was measured with a HACH-pH meter. The effects of various parameters on the rate of adsorption process were observed by varying contact time, adsorbent concentration, initial Cr Concentration, and pH of the solution. The solution volume (ïÿýïÿý) was kept constant. The measurements were made at the wavelength λ=540nm, which corresponds to maximum absorbance [13]. Using a mass balance, the concentrations of chromium (VI) at different time adsorbed in soil solids was calculated, |
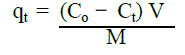 |
| where qt is the amount of chromium (VI) adsorbed onto the natural soil at time t, Co is the initial concentration of chromium (VI), Ct is aqueous phase concentration of chromium (VI) at time t, V is the volume of the aqueous phase, M is the weight of natural soil. |
III. RESULT AND DISCUSSION |
| A. Effect of solution pH |
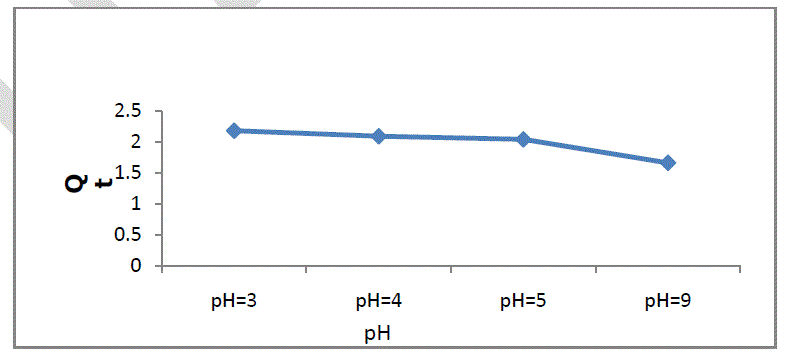
| The pH of aqueous solution plays an important role in the adsorption capacity. The effect of pH on adsorption of Cr onto soil was studied at pH 3.0 to 9.0, and the maximum adsorption capacity of soil was found to be at pH 3.0. Figure 1 shows the adsorption of chromium with pH, and it can be seen from the figure that chromium adsorption decreased with increasing pH value in the range of 3-9. And very slightly changes change between pH 4-5.The reason for this decrease can be explained by the fact that the dominant form of Cr (VI) at initial pH 3 was HCrO4 - and increase in pH shifts concentration of HCrO4 - to other forms, i.e. CrO4 - and Cr2O7 -2.The increase in Cr sorption at acidic pH could be attributed to strong electrostatic attraction between positively charged groups of soil(i.e. Ca+2 and negatively charged HCrO4 - ions. However, the decrease in adsorption due to increase in pH may be due to result of decrease of electrostatic attraction, and competitiveness between Cr anionic species (CrO4 -, Cr2O7 -2) and the OH- ions in the bulk for sorption on active sites of soil. The slight decrease in adsorption by changing pH from 4 to 5 can be attributed to neutral form of chromium [15]. |
| B. Effect of soil amount |
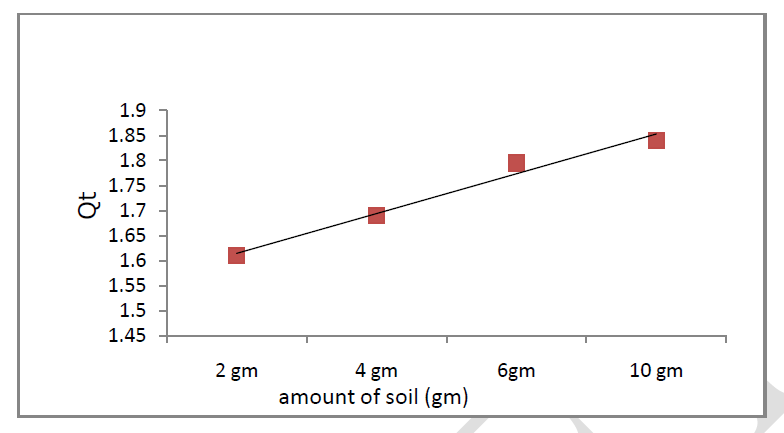
| The effect of amount of soil ranging from 2-10 gm on the Cr (VI) adsorption is shown in Figure 2. The adsorption of Cr (VI) increased with amount of soil up to 10 gm, this is due to the fact that a different soil dose has a different number of sorption centres. Cr (VI) adsorption efficiency was increased with increase in soil amount [14]. |
 |
 |
| C. Effect of initial Cr (VI) concentration |
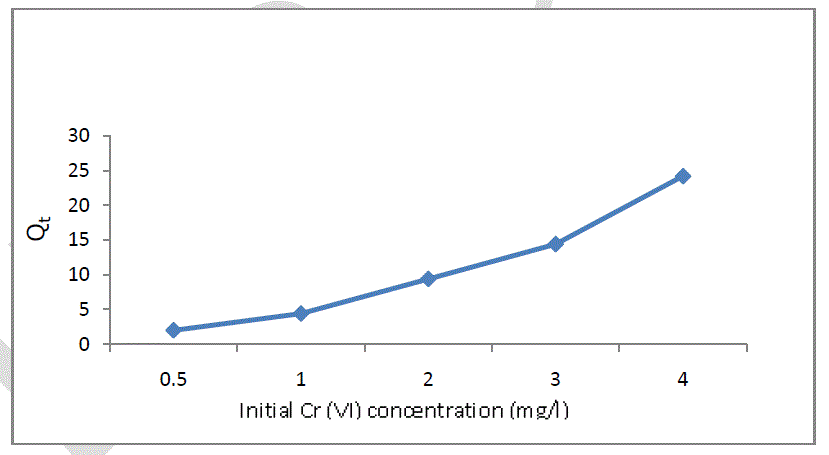
| The effect of different initial concentration of Chromium (VI) onto soil is presented in figure 3. Five different concentrations of 0.5, 1, 2, 3, and 4 mg/l for Cr (VI) were selected to investigate the effect of initial concentration of Cr (VI) onto soil. The temperature was maintained at 30oC and the amount of Soil 2 gm. It was found that the adsorption increases with increase in initial concentration of Chromium (VI). Which may be due to the availability of more number of Cr (VI) ions in solution for adsorption to the available sites [9]. |
 |
| D. Effect of Contact time |
| The effect of contact time on the adsorption of Cr (VI) was investigated. Contact time is one of the most effective factors in batch adsorption process. In order to determine equilibrium time for soil on the adsorption efficiency of chromium ions, we studied to change of adsorption capacity with time in the chromium concentration of 4 mg/L. In Figure 4, it is presented the influence of contact time on sorption process. It can be observed that in about 60 minutes the equilibrium is reached and after that, the final concentration remain constant. |
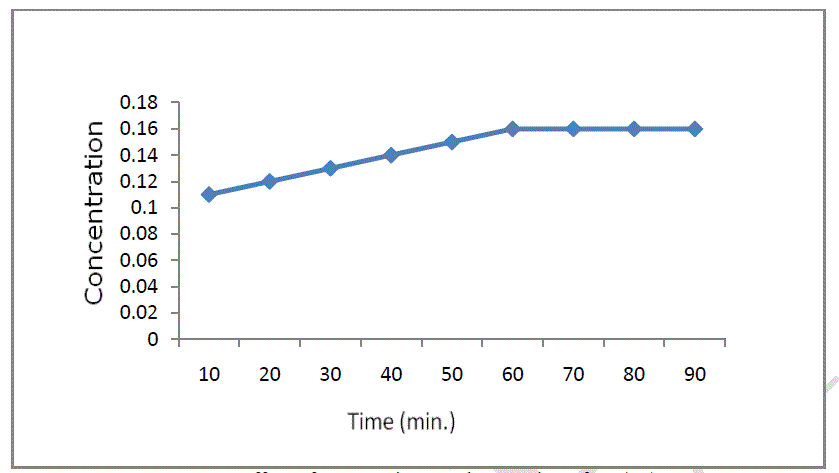 |
| E. Effect of particle size |
| Soil particle size has a significant influence on pollutants sorption from aqueous solutions because of different distribution of active centres. In this study 3 fraction were used with particle dimensions of: 100 BSS, 200 BSS and 300 BSS. In Figure 5 it is presented the influence of particles size on Cr (VI) sorption. It can be seen that the uptake is higher on small size soil particles [12]. |
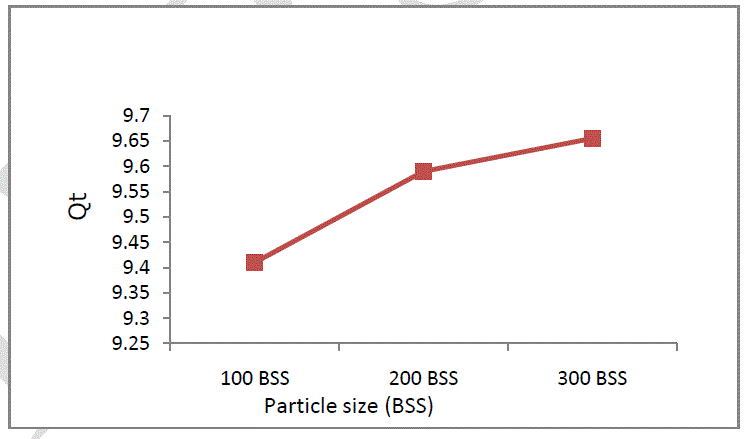 |
| F. Adsorpion isotherms |
| Adsorption is usually described through isotherms, that is, functions which connect the amount of adsorbate on the adsorbent. Distribution of metal ions between the liquid phase and the solid phase can be described by several isotherm models such as Langmuir and Freundlich [1]. The Langmuir model assumes that uptake of sorbate occurs on a homogenous surface by monolayer sorption without any interaction between the sorbed ions. Also, all the binding sites of surface have equal energy of sorption. The linear form of the Langmuir equation can be given as: |
| Ce/qe = 1/bqe + Ce/qe (2) |
| where qe; is the monolayer adsorption capacity (mg/g), b; is the Langmuir constant (L/mg), Ce; is equilibrium concentration of sorbate. The plot of Ce/qe versus Ce was employed to calculate the intercept value of 1/bqe and slope of 1/qe as shown in figure 6. One of the essential characteristics of this model can be expressed in terms of the dimensionless constant separation factor for equilibrium parameter, RL, defined as: |
| RL = 1/1+bC0 (3) |
| The value of RL indicates the type of isotherm to be irreversible (RL=0), favourable (0<RL<1), linear RL=1 or unfavorable RL>1. |
| The Freundlich isotherm, on the other hand, assumes o heterogeneous sorption surface with sites that have different energies of sorption. This model can be presented as: |
| lnKf = lnKf + 1/nf*Ce (4) |
| Where Kf; is the relative sorption capacity of sorbent, and nf; is a constant related to sorption intensity. The plot of lnqe versus lnCe should give a straight line with a slope of 1/nf and intercept of lnKf . To evaluate the applicability of Langmuir and Freundlich isotherm models for the adsorption of Cr(VI) ions by plane leaves, all parameters of these models were calculated and are shown in Table 1. |
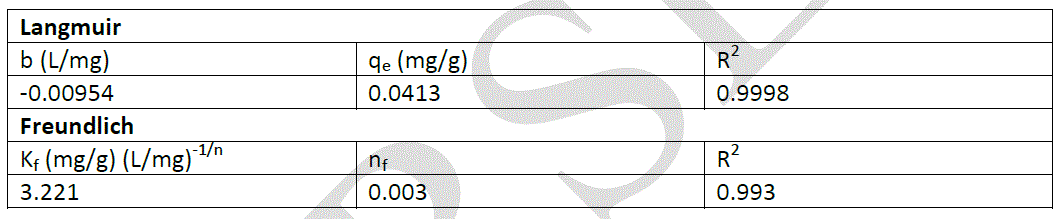 |
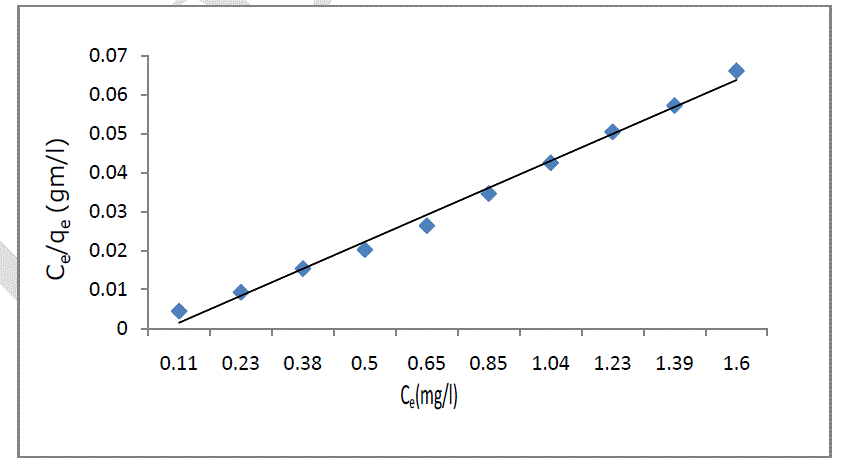 |
IV. CONCLUSION |
| The aim of this work was to study adsorption desorption of Chromium(VI) from aqueous solution to dye contaminated soil. This adsorption process is a function of particle size, amount of adsorbent, contact time, initial chromium concentration and pH of solution. The result indicate that the adsorption increases with decreasing particle size, with increasing amount of soil and initial chromium concentration. A contact time of 60 min. was found to be optimum. Adsorption is maximum on pH 3 and decreases with increasing pH. The equilibrium data fitted better with the Langmuir isotherm than Freundlich isotherm . The present study will helpful to understand the various parameter which affect the migration of Chromium in the dye contaminated area. |
References |
|