E- ISSN: 2320 - 3528
P- ISSN: 2347 - 2286
E- ISSN: 2320 - 3528
P- ISSN: 2347 - 2286
Saravanan P1 and Sathish Kumar S2*
Department of Biotechnology, St. Joseph’s College (Autonomous), Trichy- 620002, Tamil Nadu, India
Department of Botany, St. Joseph’s College (Autonomous), Trichy- 620002, Tamil Nadu, India
Received date: 28/12/2012 Revised date: 01/01/2013 Accepted date: 04/01/2013
Visit for more related articles at Research & Reviews: Journal of Microbiology and Biotechnology
Bioaccumulation of zinc (II) by yeast isolate C. krusei was investigated in different growth media. The isolate showed maximum bioaccumulation of zinc (II) in the medium prepared from sugarcane bagasse extract. The growth and bioaccumulation properties of yeast were tested as a function of pH and initial metal concentration in bagasse extract. The optimum pH value for growth and metal accumulation was determined as 5.5. The combined effects of sugar extracted from bagasse and initial zinc (II) concentration on specific growth rate and bioaccumulation efficiency of yeast was investigated. At a constant zinc (II) concentration, the growth and zinc (II) bioaccumulation increased with increasing concentrations of sugar up to 20 g/L. The inhibition effect of zinc (II) ions on the specific growth rate of yeasts was studied by non competitive and uncompetitive inhibition models at various concentrations of zinc (II) ranging from 0-25 mg/L at constant sugar concentrations (5- 20 g/L).
Bioaccumulation, zinc (II), Yeast, Sugarcane bagasse extract, Inhibition model.
Zinc is naturally released into the environment by various industrial activities like mining, galvanizing, electroplating, zinc refining, steel production and solid waste incineration, ceramics, textiles, fertilizers, pigments and batteries (USDHHS 1993). Water reservoirs are contaminated by the run-off from these industries. Although zinc is required in low quantity for growth and metabolism for lives but it is toxic above the permissible limit, 5 mg/L as they cause accumulative poisoning, cancer and brain damage[1]. Galvanized zinc containers in food preparation showed the symptoms of nausea, vomiting, and diarrhea, sometimes accompanied by bleeding and abdominal cramps.
Conventional methods of zinc removal (chemical precipitation, ion exchange and electrochemical deposition) are inefficient due to high cost and disposal problems of the solid waste generated from such treatment. Therefore, bioremoval of zinc (II) ions by using microbes has been considered more suitable than existing chemical methods. Metal uptake by yeasts is biphasic, involving rapid, metabolism-independent binding to cell surfaces, followed by a slower phase of metabolism- dependent intracellular uptake [2,3]. Reports are scanty regarding the efficiency of living yeast for removal of zinc (II) ions.
Kinetic Approach
Two Inhibitions models viz. Non competitive and Uncompetitive were tested in order to determine the toxic effects of zinc (II) ions on the specific growth rate and metal accumulation and are classified according to the effects of toxic compounds on the specific growth rate and saturation constant Ks, following the rate expressions of the models [4].
The objectives of the present study were: 1) to study the effect of various physico-chemical parameters viz. growth media and pH on growth and bioaccumulation properties of the yeast C. krusei in batch system. 2) To identify the combined effect of sugarcane bagasse extract and zinc (II) on the growth and bioaccumulation properties of the yeast isolate and 3) to typify the inhibition of zinc (II) ion on yeast growth through modeling.
Metal Solution
Zinc (II) stock solution was prepared (1000 mg/L) by dissolving 4.55 g of powdered Zn(NO3)2.6H2O (Hi Media, Mumbai, India) in 1000 mL of deionised water. The working solutions of metal were prepared by diluting the stock solution to desired concentrations.
Microorganism and Growth Conditions
C. krusei was isolated from electroplating wastewater collected from Krishna electroplating works, Kolkata, West Bengal. The isolate was phenotypically characterized and identified to species levels by Viktek 2 Compact yeast card reader with software version V2C 03.01 at the Council for Food Research and Development (CFRD), Kerela, India. The isolate was sub cultured in YEPD agar slant and maintained at 4 °C. Various concentration of extracts (10 %, 20 %, 30 %) were prepared by boiling different amount of bagasse (10 g, 20 g, 30 g) in 100 mL of distilled water respectively. The total sugar content were analysed 8 g/L ,16 g/L, 24 g/L for 10 % ,20 % and 30 % respectively by anthrone method. The aqueous extract of sugarcane bagasse was prepared following the method [5].
Bioaccumulation Assay
An aliquot of 2 mL of exponential phase yeast culture was transferred to 100 mL of aqueous extract. The effect of growth media on bioaccumulation of zinc (II) ion was studied by transferring 2 mL of the exponential phase yeast culture to various growth media viz. Mineral salt medium (MSM), Yeast extract peptone dextrose (YEPD), Malt extract glucose (MEG) and sugarcane bagasse extract supplemented with 10 mg/L of zinc (II) ion at pH 5.0. The composition of sugarcane bagasse extract (10%) was reported in our previous study [4]. The effect of pH on bioaccumulation of zinc (II) ion was investigated by varying the pH (3-7) of the aqueous extract. The combined effect of media concentration and zinc (II) concentration was studied by varying the metal concentration from 10-50 mg/L at constant aqueous extract concentrations (8, 16, 24 g/L).Cultures were grown 25 °C on a rotary shaker at 120 rpm. Samples were taken at regular time intervals and centrifuged at 10,000 rpm for 5 min. The pellet was dried at 40 °C to a constant weight for biomass estimation and the supernatant was analyzed for residual metal concentration by reading the absorbance at 213.9 nm using AAS (Varian AA 240, Australia). All the experiments were carried out in triplicates. The metal uptake values were determined as follows:
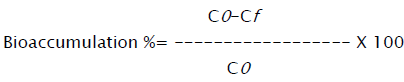
Where C0 is the initial concentration of metal (mg/L). Cf is the final concentration of metal (mg/L).
Growth and zinc (II) bioaccumulation properties of C. krusei was studied as a function of pH and initial metal concentration. Combined effect of sugarcane bagasse extract and zinc (II) on the growth and bioaccumulation properties of C. krusei were also investigated. The results were given as the units of bioaccumulation percentages at the end of the growth, biomass dry weight (X; g/L) and specific growth rate of the yeasts (μ: 1/h).
Effect of Growth Media on Growth and Zinc (II) Bioaccumulation
The bioaccumulation of zinc (II) by C. krusei was studied using different growth media viz. MSM, YEPD, MEG and aqueous extract of sugarcane bagasse medium. Table 1 showed that the specific growth rate and zinc (II) bioaccumulation properties of C. krusei was highly influenced by growth media. The maximum zinc (II) bioaccumulation by the yeast isolate was noted in medium containing sugarcane bagasse extarct due to its high sucrose content which increased the biomass production resulting in higher zinc (II) bioaccumulation C. krusei showed maximal growth (0.1186 1/h) and zinc (II)accumulation (68 %) in sugarcane bagasse medium respectively.
Effect of Initial Ph on the Growth and Zinc (II) Bioaccumulation of C. krusei
The comparative growth and zinc (II) bioaccumulation properties of C. krusei were investigated as a function of initial pH, initial sugar concentration (8 g/L) and initial zinc (II) ion concentrations (10 mg/L). Table 2 (a,b) showed that maximum bioaccumulation occurred at an optimum pH 5.0.
The growth of yeasts C. krusei were studied at an initial pH of 5.0 with increasing bagasse extract concentrations in the absence and presence of a constant zinc (II) ion concentration. Keeping the initial metal concentration constant between 10 to 50 mg/L, the initial concentration of sugar extracted from bagasse was changed from 8 to 24 g/L. Bioaccumulation %, specific growth rate and biomass dry weight of C. krusei at different concentration of metal with increasing sugar concentration were compared in Table 3. Higher growth rates were obtained in media without metal. When sugar concentration was kept constant and initial zinc (II) concentration was changed from 10 mg/L to 50 mg/L, growth rates of yeasts were reduced. The presence of zinc (II) in the growth medium repressed the growth of the microorganisms irreversibly and this inhibition effect increased with the zinc (II) ion concentrations for all initial sugar concentration.
The present study showed that the increased concentration of sugar reduced the inhibitory effect of zinc (II) on the growth of the yeasts. Similar results on increase in cell concentration and specific growth rate with increase in sugar concentration were reported by Aksu and Donmez [6] during bioaccumulation of copper (II) by Kluyveromyces marxianus using molasses medium.
Inhibition Models
In absence of zinc (II) ion, the values of μm and Ks for C. krusei were determined as 0.1811 1/h and 3.23 g/L respectively from the Monods equation by linear regression method. Non competitive inhibition model and uncompetitive model were examined to determine the metal inhibition kinetics on growth of the yeast. The plot of 1/μ vs 1/S obtained in the presence of increasing zinc (II) concentrations showed that the inhibition obeyed the non-competitive inhibition model. The μm,app, could be determined from the intercept of linear plot of at different initial metal concentration. The values of inhibition constant, KI were calculated from determined μm,app, and known initial metal concentrations values. The values of inhibition constant indicate the tolerance level of yeast isolates. Table 4 showed the noncompetitive and uncompetitive model parameters for bioaccumulation of zinc (II) by C. krusei respectively.
According to noncompetitive and uncompetitive inhibition models, the Ks values should decrease at higher concentration of pollutants [7]. Non competitive model was best fitted to the experimental data with significant decrease in Ks values. The inhibition constants of both the models were found to increase with increasing zinc (II) concentration indicating the toxicity of metal at higher concentrations. At higher zinc (II) concentration (50 mg/L), the inhibition constant for C. krusei was found as 59.36 mg/L for noncompetitive model and 66.54 mg/L for uncompetitive model.
The effects of zinc (II) ions on the growth and metal accumulation properties of C. krusei was studied in a batch system as a function of growth media and pH. The results showed that the percentage of zinc (II) accumulation was dependent on growth media and pH. The obtained results also indicated that C. krusei is capable of accumulating zinc (II) using extract of sugarcane bagasse, which served as the sole source of nutrient in the medium. Bioaccumulation percentages of zinc (II) was increased with increasing concentration of sugar extracted from sugarcane bagasse which reduced the inhibition effect of zinc (II) on yeast growth.