e-ISSN: 2321-6182 p-ISSN: 2347-2332
e-ISSN: 2321-6182 p-ISSN: 2347-2332
Judith Uchechi Anyaba*, Ovgu Isbilen and Ender Volkan
Department of Pharmacognosy, Cyprus International University, Haspolat, Cyprus
Received: 10-Apr-2023, Manuscript No. JPRPC-23-94926; Editor assigned: 12-Apr-2023, Pre QC No. JPRPC-23-94926 (PQ); Reviewed: 26-Apr-2023, QC No. JPRPC-23-94926; Revised: 09-Jul-2023, Manuscript No. JPRPC-23-94926 (R); Published: 16-Jul-2023, DOI: 10.4172/2321-6182.11.4.001
Citation: Anyaba JU, et al. Bioactivity and Phytochemical Screening of Sideritis Cypria Wild Plant. RRJ Pharmacogn
Phytochem. 2023;11:001.
Copyright: © 2023 Anyaba JU, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Research & Reviews: Journal of Pharmacognosy and Phytochemistry
Sideritis cypria (S.cypria) is an endangered species that is endemic to the Turkish Republic of Northern Cyprus (TRNC). This study investigated some of the microscopical and macroscopical characteristics, the antimicrobial and the bioactive phytochemical components of the leaves and flowers of the S.cypria. The main aim of the study is to provide additional literature to the already available literature on S.cypria by determining the anatomical and bioactive properties of the wild plant, and also determining the plant part with the highest level of bioactivity. Green and brown leaf and flower samples were macerated in different solvents and used for analyses. Anatomical results showed the presence of glandular and covering trichomes, epithelial cells with guard cells, and parenchymal cells. Antimicrobial results showed significant activity against E. coli, Meticllin Resistant S. aureus, Salmonella, B. subtilis and E. faecalis by both samples of leaves and flowers, with higher activity on the Brown leaf/Acetone and flower/petroleum ether extract. For the phytochemical screening results, 52 bioactive phytoconstituents were isolated from 8 extracts of both leaves and 2 extracts of the flowers of the wild plant which include; 15 terpenoids, and 14 alkanes, 7 alkaloids, 7 phenols, 4 fatty acids, 2 flavonoids, and 1 steroid. The brown leaf/acetone extracts and the flower/methanol gave the highest yield of phytoconstituents amongst all other extracts.
Sideritis cypria; Endemic plants; Bioactivity; Phytoconstituents; Herbal drugs; GCMS
For so many years with the aid of technology, scientists have focused on artificial/synthetic methods to produce drugs in contrast to the older traditional medicine in which natural sources of drugs were used for their therapeutic benefits. Most of these synthetic drugs were produced by chemically synthesizing new compounds for their therapeutic benefits. The use of synthetic drugs is associated with many side effects and adverse drug reactions and this precipitated the need for alternative and less toxic sources of drugs. In the U.S, 8% of hospital admissions are due to adverse drug reactions or side effects of synthetic drugs. Little or none of such events are being reported for natural drugs. Naturally produced bioactive compounds have been shown to have lesser or no side effects. This has increased the search for newer, more effective, and natural antimicrobials that can be used to fight off these resistant pathogens. The impact this has made in the science field is seen in the current worldwide interest in the discovery and refining of natural sources of drugs [1].
One of these plant sources is the Sideritis cypria, a wild plant endemic to North Cyprus, which has been analyzed severally for its intrinsic properties. The Sideritis species is one of the numerous geni of the Lamiaceae family. The name is gotten from the Greek word ‘σίδερο’ which means ‘iron’, because some species were used to treat wounds caused by metal weapons [2]. Sideritis species have been traditionally used in the treatment of gastrointestinal conditions and other inflammatory conditions for ages and for this purpose, has been approved by the EMA for use. Sideritis species grow annually or perennially and prefer rocky and sunny slopes from sea level to more than 3000 meters for vegetation. Their leaves are narrow, opposite, entire or crenate dentate, sessile to petiolate. Flowers form a false whorl of inflorescences around the nodes, also known as verticillasters. Sideritis species attain their optimal growth in the fall sun, are well adapted to drought conditions, and, require slightly alkaline soil rich in nutrients. There are over 150 Sideritis species widely distributed across the Mediterranean region, in the Marmara and Aegean regions of Turkey, in the Southeast of the Iberian Peninsula, and the Canary Islands. 40 of the Sideritis taxa are endemic in Turkey. In particular, three Sideritis species have been found in Cyprus; S. curvidens Stapf, S. perfoliata L., and the endemic S. cypria post [3].
The habitat
Sideritis cypria is a small shrub measuring 34.5 cm in height. The distribution of Sideritis cypria in North Cyprus is sparse because of its preferred habitat on rocky soil between the rock clefts or dry limestone cliffs facing South, under sufficient exposure to sunlight (Figure 1). For its conservation, species seeds are kept at the agricultural research institute, national gene bank [4].
However, the S. cypria has only just begun to attract the interest of researchers, and about three to four studies on this species phytochemical properties were published in the last five years. S. Cypria is gaining attention due to the therapeutic benefit attributed to the Sideritis species, and because it has been discovered to contain a wide range of bioactive compounds by recent studies [5]. The Bioactive effects of S. cypria are attributed to its diterpenoid, flavonoid, and essential oil content. The majority of the studies are focused on the essential oils of the cultivated plant and there is not much literature on the bioactive capabilities of the plant leaf and flowers. Also, there has not been much literature focused on determining the solvent type that will give the highest yield of bioactive phytoconstituents in the S. cypria. Additionally, a comparison between two types of the same plant part such as the brown and green leaf, has never been considered in previous literature. This study aims to discover the bioactivity of the three plant materials of the S. cypria and the solvent type that will yield the highest level of phytoconstituents [6].
Plant collection, drying and storage
Sparse quantity of S. cypria plant materials were collected from the Western part of the pentadaktylos mountain range at latitude 35.312525 and longitude 33.281221 in the Kyrenia town of TRNC at an altitude of 725 m above sea level at midday at 16°C temperature on 26th November 2021. The plants were snipped at the base above the roots to preserve the roots and encourage conservation of species. Collected materials were taken back miles away to the research laboratory of the faculty of pharmacy at Cyprus international university, TRNC. Materials were dried at room temperature, weighed, grounded to powder, and stored in paper sheets [7].
Macroscopical evaluation: The macroscopical studies of a plant include the observation of morphological and organoleptic characteristics through the naked eye. Dried aerial parts of the plant were observed for organoleptic characteristics such as colour, taste, odour, size, and shape [8].
Microscopical evaluation: Each of the dried flower and leaf samples was sectioned with a razor blade and fixed on a microscope slide and cleared with 2 drops of chloral hydrate or hydrochloric acid. The samples were then placed to heat very briefly to release air bubbles and afterwards observed under a binocular microscope at different magnifications. Relevant photographs were taken with an iPhone digital camera. Detailed and micro views of certain organoleptic structures were obtained using the JEOL JSM-6610LV scanning electron microscope [9].
Preliminary phytochemical screening: The chemical tests for both primary and secondary phytoconstituents in the dried powder and extracts of Sideritis cypria were carried out as described below [10].
Detection of alkaloids:
• Dragendorff’s test: Each sample of leaf and flower powder was dissolved in 5 mls of distilled water, 2 mls of 2M HCL, and 1 ml of dragendorff’s reagent was added and examined for the formation of an orange-red precipitate [11].
• Mayer’s test: Each Sample of leaf and flower powder was mixed with a few drops of dilute HCL and Mayer’s reagent and was observed for the formation of a white precipitate [12].
Detection of glycosides:
• Borntrager’s test: Each sample of leaf and flower powder was boiled in 1 ml of 1M sulphuric acid in a test tube for five minutes, and filtered while hot and then cooled. An equal volume of chloroform was added and the solution was shaken. The lower/ammoniacal layer of the solvent was collected and mixed with a few ml of dilute ammonia solution and observed for the formation of a rose pink to red colour [13].
Detection of steroids and triterpenoids
• Salkowski test: Few drops of concentrated sulphuric acid were added to the plant extract and observed for the formation of a red colour (if steroids are present) or yellow colour (if triterpenoids are present) at the lower layer [14].
Detection of flavonoids
• Alkaline reagent test: A few drops of sodium hydroxide solution were added to the solution of the samples to test sample solutions to observe the formation of an intense yellow colour which turns colourless when a few drops of dilute acid are added [15].
Detection of carbohydrates
• Fehling’s test: Fehling's reagent was mixed with solutions of the samples. This was heated and examined for the appearance of red colouration [16].
Detection of phenols
• Ferric chloride test: a little amount of the different samples were dissolved with 2 ml of distilled water and a few drops of 10% aqueous ferric chloride solution were added and observed for the appearance of a blue/green colour.
Detection of proteins
• Biuret test: 5 to 8 drops of 10% copper sulfate solution was added to samples to observe for the formation of a violet colour [17].
Detection of tannins
A few drops of 5% aqueous ferric chloride solution were added to 2 ml of an aqueous extract of the drug and examined for the appearance of a bluish black colour [18].
Detection of saponins
A drop of bicarbonate solution was added to the samples and shaken vigorously and then left for 3 minutes. The samples were observed for any honey comb like froth.
Detection of fixed oils and fats
Small quantities of plant samples were placed on a filter paper, folded in, and pressed to observe for any oily stain on the filter paper [19].
Chemical profiling
Samples to be analyzed were prepared by maceratıng 0.1 g of powder samples in 100 ml of both polar and nonpolar organic solvents. For chemical profiling, the following extracts were prepared;
Flower: Methanol and petroleum ether extracts.
Green leaf: Methanol, petroleum ether, ethyl ether, and acetone extracts.
Brown leaf: Methanol, petroleum ether, ethyl ether, and acetone extracts [20].
Samples were macerated for two days on a see saw rocker (Stuart SSL4) at 43 oscillations per minute at room temperature.
Fourier-transform infrared spectroscopy
FTIR is the most common form of infrared spectroscopy. It identifies the infrared spectrum of absorption, emission and photoconductivity of the components of matter. Different functional groups present within a sample can be identified according to the different ranges of absorption of infrared radiation between the ranges of 400 to 4000 cm-1 on the infrared spectrum. In the FTIR analysis, the various samples of the green leaf, brown leaf and the flower powder and extracts were subjected to contact with infrared radiation using the IR Prestige-21 SHIMDAZU Fourier-Transform Infrared Spectrophotometer.
Gas chromatograph-mass spectrometry
GCMS is useful in separating and identifying chemical compounds present within a plant sample at a molecular level. Macerated samples were filtered off initially with filter paper, and about 2 mls of the filtrate was re-filtered with a syringe filter (ISOLAB laborgerate GmbH, non-sterile RC-45/25 mm) for GCMS analysis. For analysis, samples were placed in Auto injector vial wells (AOC-20; Autoinjector SHIMADZU) and run for 52 minutes. The oven temperature was kept at 50ºC for 2 min and increased to 250ºC. The carrier gas Helium at a flow rate of 1 mL/min facilitated the transmission of each volatile compound across the separating column where it was isolated and afterwards, identified by mass spectrometry (GCMS-QP2010 plus gas chromatograph mass spectrometer-shimdazu).
Antimicrobial analysis
For this study, the antimicrobial activity of the S. cypria was analyzed using the disk diffusion by Kirby-Bauer method. Additionally, in order to optimize the results, different solvents were used to prepare different extracts so as to determine the extract with the greatest antimicrobial activity.
Preparation of plant extract: Samples to be analyzed were prepared by macerating 0.5 g (BL and GL powders) and 0.25 g (flower powder) 500 mls and 250 mls of organic solvents respectively, on a see-saw rocker at 43 oscillations per minute at room temperature for 48 hours. Macerated samples were afterwards evaporated to 50 mls under the hood by using a water bath at 40ºC, transferred to sterile falcon tubes and further evaporated to dryness. The following samples were used for the antimicrobial analysis of the S. cypria.
Flower: Petroleum ether extracts.
Green leaf: Petroleum ether, methanol, and acetone extracts.
Brown leaf: Petroleum ether and acetone extracts.
In order to bring the extract to the solution, dried extracts were concentrated with 1% PBS and stored in the refrigerator. The blank disks used for the disk diffusion assay were cut out from filter paper using a paper hole puncher of 6 mm diameter and sterilized in the autoclave at 140ºC-160ºC for 3 hours before use.
Organisms and growth conditions: The microorganisms used for this study were retrieved from the culture collections of the department of pharmacy at the Cyprus international university. Five bacterial cultures including both gram positive and gram negative bacteria (Salmonella, Escherichia coli, Bacillus subtilis, Enterococcus faecalis, and Staphylococcus aureus) were obtained for this study. The microorganisms were selectively cultured in Mueller Hinton Agar (MHA) in the incubator at 37ºC for 24 hours and afterwards, stored in the refrigerator for research uses.
Disk diffusion assay-Kirby Bauer method: While maintaining a sterile field, bacterial cultures were inoculated onto a Mueller Hinton agar plate and allowed to dry for a few minutes. Sterile paper disks were soaked in 50 μl of micro filtered plant extract and placed on the agar plate. For positive control, ciprofloxacin, which has a broad spectrum against both gram positive and gram negative bacteria was the chosen antimicrobial, and for the negative control, PBS (Phosphate Buffer Saline) soaked disks were applied. Culture disks were incubated for 12-18 hours at 37ºC. The radius of the zone of inhibition was obtained by measuring the distance from the edge of the inhibition zone/wall to the centre of the disk containing the plant extract. The antimicrobial test was done in triplicate for standardization and accuracy. The standard mean of error was obtained for each positive set of trials.
Statistical analysis: All experiments were performed three times in triplicates. Statistical significance was determined via Students’t-test. Data represented as ± SEM.
Macroscopical charateristics of whole plant parts
Leaves: The leaves measure about 6 cm in length and 1.7 cm in width, simple, green/brown, and oblanceolate with a basal arrangement and pinnate venation (Figure 2). The leaf margins are ciliated with a densely lanate surface; have a rounded apex with a tapering/cuneate base.
Flowers: The flowers of the Sideritis cypria are yellow with a slightly aromatic smell. Verticillasters or secondary inflorescence is about 4-6, mostly distant and rarely crowded (Figure 3). The middle bracts
Are equal or slightly taller than or equal to the flowers with a suborbicular central portion. There is no visible sepal/calyx. The corolla is bright yellow and about three of them are fused at the base to form a tubular pattern.
Macroscopical characteristics of powdered green and brown leaves
The organoleptic characteristics of the plant powder samples were observed to differ slightly between the green leaf and the brown leaf powder samples in colour only.
The green leaf powder: colour: Green. Odour: Aromatic. Shape: round. Texture: woolly/coarse. The brown leaf powder: colour: Brown. Odour: Aromatic. Shape: Round. Texture: Woolly/Coarse. The flowers: Colour: Yellow. Odour: Aromatic. Shape: Cylindrical. Texture: Rough (Figure 4).
Microscopical characteristics
Microscopical observation of the flower reveal round and polygonal epidermal cells with multiple guard cells both on LM and SEM with a better view of the closed stomata on the SEM view. Abundant, simple, unicellular, uniseptate, and thick walled fibres can also be seen either single or multiple. Several uniseriate, uni/multicellular covering trichomes are also evident with bases arising from the epidermis (Figure 5).
Figure 5: Light microscopy of flower powder showing guard cells and stoma embedded in the epidermal layer with multiple pollen grain like spherical structures; (a) Lugol stained sample showing the bluish colouration of parenchymal cell walls and the palisade cells of the upper epidermis; (b) Lugol stained section showing the parenchymal cells, vascular bundle, multiple guard cells, non-glandular multicellular uniseriate trichome on the left (type 5), mesophyll tissue and stained starch grains; (c) Fibrous cells; (d) X400 Magnification; SEM of the flower fiber showing multiple closed stoma.
In both green and brown leaf, micro-morphological structures such as the trichomes, epidermal cells, guard cells, stomata and palisade cells were observed using a light microscope and a Scanning Electron Microscope (SEM). The trichomes include both covering and glandular trichomes; uniseriate and multiseriate; unicellular and multicellular. Covering trichomes have a tapering apex while the glandular trichomes have an apical glandular cell. Covering trichomes can be seen arising out of the outer epidermal cell layer. Epidermal cells are mostly rounded and between them are some guard cells and the stomatal opening. The palisade and Spongy layers are also observed (Figures 6 and 7).
Figure 6: Light Microscopy of leaf samples; (a) Green leaf surface venations with numerous trichomes x100; (b) Numerous non-glandular pinnate trichomes arising from all sides of the green leaf x40; (c) Microscopy of the covering trichomes of the leaf; (d) x100 magnification of palisade cells, guard cells and multiple non-glandular and glandular trichomes around the epidermal cell layer; (e) Brown leaf surface showing the venation type and a meshwork of covering trichomes x100; (f) Unicellular uniseriate non-glandular trichome at the centre and 2-3 glandular trichomes to the right x400; 2) Diagrammatic representation of some microscopic characteristic features of the s. cypria.
Preliminary phytochemical results
The green leaf sample showed positive results for steroids, flavonoids, carbohydrates, and phenols. The brown leaf sample showed positive results for steroids, flavonoids, carbohydrates, tannins, and phenols. Flower sample also showed positive results for steroids, flavonoids, carbohydrates, and phenols (Table 1).
| Phtochemical | Green leaf powder | Sundried/Browned leaf powder | Flower powder |
|---|---|---|---|
| Alkaloids: Dragendorff’s test: Mayer’s test: |
- | - | - |
| Glycosides: Borntrager’s test: |
- | - | - |
| Steroids and triterpenoids Salkowski test: |
+ For steroids. - For triterpenoids. |
+For steroids. -For triterpenoids. |
+ For steroids. -For triterpenoids. |
| Flavonoids: Alkaline reagent test: |
+ | + | + |
| Carbohydrates: Fehling's test: |
+ | + | + |
| Phenols: Ferric chloride test: |
+ | + | + |
| Proteins: Biuret test: |
- | - | - |
| Tannins Ferric chloride test: |
- | + | - |
| Saponins | - | - | - |
| Fixed oils and fats | - | - | - |
Table 1. Quantitative phytochemical analysis of plant powder samples. – for negative. + for positive.
Antimicrobial analysis
Antimicrobial activity of each plant material showed variations with the type of solvent used. Generally, all aerial parts studied displayed a wide range of antimicrobial activity against both gram-negative and gram-positive bacteria. As a valid positive control, ciprofloxacin (5 μg) displayed the largest zone of inhibition against all five microorganisms in all trials. The negative control, PBS, had little or no antibacterial activity in all trials. The brown leaf extracts, with acetone and petroleum ether showed significant antibacterial activity. Its acetone extract was active against all bacteria except E. faecalis ATCC 29212, while its petroleum extract was active only against E. coli B29. The reason for this wide margin of difference is most likely related to the type and the level of phytoconstituents present within each extract which is directly proportional to the solvent used for extraction. The only flower extract used for the antimicrobial analysis, the petroleum ether extract, displayed a wide range of activity against all bacteria except E. coli B29. The green leaf extracts, methanol, petroleum ether, and acetone, had significant antimicrobial activity against E. faecalis, Salmonella and E. faecalis ATCC 29212 respectively. Unlike the brown leaf extracts, no green leaf extract had higher antimicrobial activity than its correspondents, but each responded against a different type of bacteria. This highlights the probability of the brown leaf having higher phytoconstituents than the green leaf, not only because of the solvent, acetone, used for extraction, but also because of the wide radius of inhibition noted with the brown leaf extracts for example, the petroleum ether extract, even to only one bacterium. The antimicrobial activity of the Flower extract also suggests a high level of bioactive compounds present within the flower of the S. cypria and the solvent used for extraction is excluded as the possible cause because other petroleum ether extracts do not display the same level of antimicrobial activity as the petroleum ether extract of the flower (Table 2 and Figure 8).
| Flower extracts | Green leaf extracts | Brown leaf extracts | ||||
|---|---|---|---|---|---|---|
| Petroleum ether | Petroleum ether | Methanol | Acetone | Acetone | Petroleum ether | |
| Salmonella | 4 ± 0 | 4.33 ± 0.167 | 0 | 0 | 4.17 ± 0.17 | 0 |
| E. coli | 0 | 0 | 0 | 0 | 3.45 ± 0.035 | 5.5 ± 0.41 |
| B. subtilis | 3.67 ± 2.4 | 0 | 0 | 0 | 6.67 ± 2.67 | 0 |
| E. faecalis ATCC 29212 | 4.83 ± 0.167 | 0 | 4.5 ± 0.47 | 4.67 ± 0.93 | 0 | 0 |
| MRSA | 5.67 ± 0.33 | 0 | 0 | 0 | 5.8 ± 0.8 | 0 |
Table 2. Mean ± standard mean of error of the radius of the zone of inhibition obtained from the antimicrobial analysis of 5 plant extracts concentrated with 6.5 ml of 1% PBS solution. 5 µg ciprofloxacin was the positive control and was active against Salmonella, Escherichia coli, Bacillus Subtilis, Enterococcus faecalis, and methicillin resistant Staphylococcus aureus with a wide radius of inhibition. All experiments were done 3 times and triplicates. Data represented as SEM.
Chemical profiling
The chemical profiling of plant extracts showed significant presence of bioactive compounds as detailed below.
Fourier transfer infrared of plant extracts: The FTIR graphs of the different plant extracts were done in order to determine the functional groups present in the plant extracts (Figure 9). Results have shown very similar absorption bands which signify a similarity in the phytoconstituents, or compounds present within the aerial parts of this plant. The functional groups present include methyl/methylene/methyne (CH3.CH2/CH), Imido (N-H), Nitrile (C≡N), Carbonyl (C=O), İmino (C=N), Alkyne (C≡C) Phenol (Ar-OH), Alcohol (OH), epoxy oxirane ring, and Silicon-Oxy-Compounds (Si-O-Si, Si-O-C).
Gas chromatograph-mass spectrometry: From the GC-MS analysis, a total of 52 bioactive phytochemicals were identified from the 8 extracts of both leaves and 2 extracts of the flowers of the wild plants which include; 15 terpenoids, 14 alkanes, 7 alkaloids, 7 phenols, 4 fatty acids, 2 flavonoids, and 1 steroid. The GL extracts; acetone, petroleum ether, methanol, and ethyl ether yielded 15, 13, 16 and 11 bioactive compounds respectively. The GL extract with the highest yield of bioactive compounds was the Methanol extract. The number of bioactive compounds present in the BL extracts, BL/A, BL/MeOH, BL/PE, and BL/EE includes 20, 13, 11, and 15 bioactive compounds respectively). The BL extract with the highest bioactive phytoconstituents is the ACETONE extract. The flower extracts; F/PE and F/MeOH yielded 14 and 17 compounds respectively, with the methanol extract having the highest number of bioactive phytoconstituents. The acetone and methanol extracts yielded the highest levels of bioactive phytoconstituents, with some compounds found to be exclusive to only the BL/A extracts. This only suggests that these compounds might be responsible for the higher level of bioactivity, specifically antimicrobial activity, in the BL/A extracts. The plant material with the highest level of phytoconstituents was gotten by comparing extracts of the same solvent together. For the acetone extracts, the BL/A>GL/A. In the methanol extracts, F/MeOH>GL/MeOH>BL>MeOH. In the Ethyl Ether extracts, BL/EE>GL>EE. Lastly, for the Petroleum Ether extracts, F/PE>GL/PE>BL/PE. In extracts prepared with all three plant materials, the flower extracts had higher values than others. This is different from the acetone and ethyl ether extracts where there was no flower extract. Therefore, in this study, the extract with the highest yield amongst all was the BL/Acetone. The plant materials with the highest yield were the brown leaf and flower samples.
Some bioactive phytoconstiutents were consistently found in most of the extracts studied. The presence of terpenoids such as octadecamethylcyclononasiloxane, dodecamethylcyclohexasiloxane, hexadecamethylcyclooctasiloxane, decamethylcyclopentasiloxane, eicosamethylcyclododecasiloxane, tetradecamethylcyclododecasiloxane, tetradecamethylcycloheptasiloxane was consistent in almost all of the extracts. These terpenoids are known antimicrobials (Figures 10-12). Di-T-Butyl-Phenol, a flavonoid with potent antimicrobial and cytotoxic activity was present in 6 of the extracts except the acetone extracts. Alkanes like dotriacontane, an antimicrobial, tetrapentacontane, an antitumor, and undecane, an antimicrobial and antioxidant, were consistently reoccurring in 7 extracts. A phenolic acid, 1,2 benzenedicarboxylic acid, which is cytotoxic, was present in 8 extracts. it is worthy of note that dotriacontane showed persistently high %area in all the almost all the extracts especially with all the brown leaf extracts (Tables 3-13).
| Retention time | Compound name | Molecular formula | Molecular weight | Biological activity | % Area |
|---|---|---|---|---|---|
| 7.852 | .Alpha.-Pinene, (-)- $$ Bicyclo(3.1.1)Hept-2-Ene, 2,6,6-Trimethyl- | C10H16 | 136.24 | Antiinflammatory, antifungal, antibacterial, antitumor, cytotoxic | 0.01 |
| 9.028 | 2-.Beta.-Pinene $$ Bicyclo(3.1.1)Heptane, 6,6-Dimethyl-2-Methylene- | C10H16 | 136.23 | Antiinflammatory, antifungal, antiibacterial, antitumor, cytotoxic | 0.03 |
| 9.651 | 1,1,3,3,5,5,7,7-Octamethyl-Cyclooctasiloxane | C8H24O4Si4 | 296.61 | Antimicrobial | 0.09 |
| 11.424 | 3-Methoxy-4-((Trimethylsilyl)Oxy)-Benzaldehyde-O-Methyloxime | C12H19NO3Si | 253.37 | 0.06 | |
| 12.839 | 2,4-Dihydroxybenzoic acid methyl ester trimethylsilyl ether | C21H3804Si2 | 410.7 | Antimicrobial | 0.05 |
| 14.434 | Decamethylcyclopentasiloxan | C10H3005Si5 | 370.77 | Antimicrobial | 0.02 |
| 17.435 | 1,3-Di-tert-butylbenzene | C14H22 | 190.32 | 0.11 | |
| 18.222 | 2,3,4-Trimethylhexane | C9H20 | 128.25 | Pharmaceutical intermediate | 0.03 |
| 19.499 | Dodecamethylcyclohexasiloxane | C12H36O6Si6 | 444.92 | Antimicrobial | 0.09 |
| 24.11 | Tetradecamethylcycloheptasiloxane | C14H42O7Si7 | 519.07 | Antimicrobial | 0.05 |
| 24.569 | 3,5-Di-tert-butylphenol | C14H22O | 206.32 | Antibiofilm and antifungal, antioxidant | 0.03 |
| 27.822 | 1,2-Benzenedicarboxylic acid | C12H14O4 | 222.24 | Cytotoxic | 0.45 |
| 27.086 | Caryophyllene oxide | C15H25O | 220.35 | Antiinflammatory, calcium-antagonist, anticarcinogenic, antioxidative, analgesic | 0.04 |
| 28.243 | Hexadecamethylcyclooctasiloxane | C16H48O8Si8 | 593.23 | Antimicrobial | 0.15 |
| 55.982 | Octadecamethylcyclononasiloxane | C18H54O9Si9 | 667.4 | Antimicrobial | 2.12 |
| 38.624 | Octadecanoic acid, methyl ester | C19H38O2 | 298.5 | Cancer preventive | 0.69 |
| 50.525 | Tetracosamethylcyclododecasiloxane | C24H72O12Si12 | 889.8 | Antimicrobial | 2.21 |
| 51.554 | Eicosamethylcyclodecasiloxane | C20H60O10Si10 | 741.84 | Antimicrobial | 0.85 |
Table 3. GCMS analysis of compounds present within the green leaf methanol extract.
| Retention time | Compound name | Molecular formula | Molecular weight | Biological activity | % Area |
|---|---|---|---|---|---|
| 8.592 | 1,3,5-trimethylbenzene | C9H12 | 120.19 | 0.31 | |
| 9.365 | 2,2,4,6,6-pentamethylheptane | C12H26 | 170.33 | 0.39 | |
| 10.102 | 5-methyldecane | C11H24 | 156.31 | 0.21 | |
| 10.516 | 1-Methyl-3-ethylbenzene | C9H12 | 120.19 | Antioxidant, antinociceptive, anxiolytic, anticancer, antinociceptive | 0.49 |
| 11.637 | Tetradecane | C14H30 | 198.39 | Antibacterial, diuretic, antituberculosis | 0.48 |
| 15.9 | Dodecane | C12H26 | 170.33 | Antioxidant | 0.3 |
| 16.311 | 2,5-dimethylundecane | C11H24 | 156.31 | 0.39 | |
| 17.582 | 1,3-Di-tert-butylbenzene | C14H22 | 190.32 | 0.75 | |
| 30.374 | Hexadecane, 2,6,10,14-tetramethyl | C20H42 | 282.5 | 7.68 | |
| 18.872 | Pentadecane | C15H32 | 212.41 | Antioxidant and antimicrobial activity | 0.67 |
| 23.031 | Nonadecane | C19H40 | 268.5 | 0.47 | |
| 23.436 | 2,6-di-tert-butylquinone | C16H24O | 220.35 | Antidiabetogenic, antioxidant, antiviral, antibacterial, cytotoxic, antifungal | 0.3 |
| 25.017 | 2,4-Di-tert-butylphenol | C14H22O | 206 | Antioxidant, antiviral, antibacterial, cytotoxic, nematoid | 0.53 |
| 28.192 | Docosane | C22H46 | 310.6 | 0.38 | |
| 34.411 | 7,9-di-tert-butyl-1-oxaspiro(4.5)deca-6,9-diene-2,8-dione | C17H24O3 | 276.37 | Antimicrobial, antiviral, and antineoplastic | 0.19 |
| 51.082 | Dotriacontane | C32H66 | 450.9 | Antibacterial, antiviral, antifungal, antioxidant, antispasmodic | 5.25 |
| 55.455 | Tetracosamethylcyclododecasiloxane | C24H72O12Si12 | 889.8 | Antimicrobial | 2.92 |
| 52.895 | Octadecane | C18H38 | 254.5 | 7.24 | |
| 49.65 | Octadecamethylcyclononasiloxane | C168H54O9Si9 | 667.39 | Antimicrobial | 1.76 |
| 49.019 | Tetrapentacosan $$ Tetrapentacontane | C54H110 | 759.4 | Antitumor | 4.03 |
| 43.72 | 2,2'-Methylenebis(4-methyl-6-tert-butylphenol) | C23H32O2 | 340.5 | Antioxidant | 0.51 |
| 53.784 | Hentriacontane | C31H64 | 436.84 | Antiinflammatory, antitubercular, antitumor | 0.43 |
Table 4. GCMS analysis of compounds present within the green leaf petroleum ether extract.
| Retention time | Compound name | Molecular formula | Molecular weight | Biological activity | % Area |
|---|---|---|---|---|---|
| 8.287 | 1,3-Dioxolane, 2-(phenylmethyl) | C10H12O2 | 164.2 | Scaffold for antiviral antitumor agents | 0.64 |
| 8.944 | 2,2,3,3,5,6,6-Heptamethylheptane | C14H30 | 198.39 | 0.25 | |
| 10.367 | 3,3,5-Trimethylheptane | C10H22 | 142.282 | 0.2 | |
| 11.49 | Octane, 5-ethyl-2-methyl | C11H24 | 156.31 | 0.44 | |
| 11.661 | Undecane, 5,7-dimethyl- | C13H28 | 184.36 | Antimicrobial and antioxidant | 0.51 |
| 12.886 | Decane, 3,7-dimethyl | C12H28 | 170.33 | 0.24 | |
| 15.907 | Dodecane | C12H26 | 170.33 | Antioxidant | 0.13 |
| 17.765 | Undecane, 3,6-dimethyl- | C13H28 | 184.36 | 1.05 | |
| 17.587 | 1,3-di-tert-butylbenzene | C12H22 | 190.32 | 0.59 | |
| 19.572 | Tetradecane | C14H30 | 198.39 | Antibacterial, diuretic, antituberculosis | 0.52 |
| 28.676 | Nonadecane | C19H40 | 268.5 | 1 | |
| 25.092 | 2,4-di-tert-butylphenol | C14H22O | 206 | Antioxidant, antiviral, antibacterial, cytotoxic, nematoid | 0.93 |
| 27.267 | 1,2-Benzenedicarboxylic acid | C12H14O4 | 222.24 | Cytotoxic | 2 |
| 30.59 | 2,6,10,14-Tetramethylhexadecane | C20H42 | 282.5 | 2.78 | |
| 44.21 | Docosane | C22H46 | 310.6 | 1.29 | |
| 48 | Dotriacontane | C32H66 | 450.9 | Antibacterial, antiviral, antifungal, antioxidant, antispasmodic | 4.47 |
| 34.414 | 7,9-di-tert-butyl-1-oxaspiro[4.5]deca-6,9-diene-2,8-dione | C17H24O3 | 276.37 | Antimicrobial, antiviral, antineoplastic | 0.11 |
| 35.903 | Octadecane | C18H28 | 254.5 | 0.15 | |
| 42.222 | Tetracosane | C24H50 | 338.65 | Potent inhibitor of B-amyloid aggregation in Alzheimer's disease | 0.49 |
| 45.122 | O O'-biphenol, 4,4',6,6'-Tetra-T-Butyl | C28H42O2 | 410.6 | 1.04 | |
| 43.75 | 2,2'-methylenebis(4-methyl-6-tert-butylphenol) | C26H34O3 | 394.55 | Antioxidant | 0.38 |
| 51.87 | Tetrapentacosan $$ tetrapentacontane | C54H110 | 759.4 | Antitumor | 1.6 |
| 47.6 | 1,3,5-Trisilacyclohexane | C6H6Si3 | 126.33 | 0.18 | |
| 52.82 | Hexacontane | C60H122 | 843.6 | 0.43 |
Table 5. GCMS analysis of compounds present within the green leaf ethyl ether extract.
| Retention time | Compound name | Molecular formula | Molecular weight | Bioactivity | % Area |
|---|---|---|---|---|---|
| 7.536 | Isopropylbenzene | C9H12 | 120.19 | Antioxidant, antinociceptive, anxiolytic, anticancer, antinociceptive | 0.13 |
| 8.557 | 2,7-Dimethyloctane | C10H22 | 142.28 | Pheromone | 0.06 |
| 8.921 | 2,2,3,3-Tetramethylhexane | C9H20 | 128.25 | 0.13 | |
| 9.671 | 2-N-Hexyl-1-D1-Aziridine | CH2NHCH2 | 43.07 | Scaffold for bioactive compounds-pyrazinoindole derivatives | 0.09 |
| 10.359 | 3,3,5-Trimethylheptane | C10H22 | 142 | 0.16 | |
| 11.459 | Undecane, | C13H26 | 184.36 | 0.26 | |
| 14.391 | Cyclopentasiloxane, decamethyl-R | C10H30O5Si5 | 370.77 | 0.04 | |
| 19.55 | Pseudoephedrine | C10H15NO | 165.23 | Decongestant | 0.07 |
| 19.448 | Dodecamethylcyclohexasiloxane | C12H36O6Si6 | 444.92 | Antimicrobial | 0.07 |
| 22.808 | 4-T-butoxy-1-methyl-1,2,3,6,-tetrahydropyridine | C19H17NO2 | 183.25 | Pharmacophore for bioactive derivatives | 0.05 |
| 37.937 | Tetradecamethylcycloheptasiloxane | C14H42Si7O7 | 519.07 | Antimicrobial | 0.25 |
| 29.54 | 2,6,10,14-Tetramethylhexadecane | C20H42 | 282.5 | 0.74 | |
| 27.853 | 1,2-Benzenedicarboxylic acid, | C12H14O4 | 222.24 | Cytotoxic | 0.13 |
| 38.326 | Octadecane | C18H28 | 254.9 | 0.5 | |
| 31.82 | 1,1,1,5,7,7,7-heptamethyl-3,3-bis(trimethylsiloxy)tetrasiloxane | C13H39O5Si6 | 443.96 | 0.06 | |
| 35.018 | Docosane | C22H46 | 310.6 | 0.43 | |
| 33.516 | Dotriacontane | C32H66 | 450.9 | Antibacterial, antiviral, antifungal, antioxidant, antispasmodic | 0.12 |
| 34.562 | 7,9-di-tert-butyl-1-oxaspiro(4.5)deca-6,9-diene-2,8-dione | C17H24O3 | 276.37 | Antimicrobial, antiviral, antineoplastic | 0.43 |
| 36.025 | 2-(2,2-dimethyl-propionyl)-5,5-dimethyl-cyclohexanone | C13H22O2 | 210.32 | 0.04 | |
| 47.486 | Octadecamethylcyclononasiloxane | C18H52O9Si9 | 667.39 | Antimicrobial | 0.44 |
| 43.849 | )2,2'-methylenebis(4-methyl-6-tert-butylphenol) | C26H34O3 | 394.55 | Antioxidant | 0.5 |
| 47.877 | Bis-O-(hexadecyl)trimetthylsilylglycerol | C11H17NO2 | 195.26 | 0.54 | |
| 48.756 | Tetrapentacosan $$ tetrapentacontan | C54H110 | 759.4 | Antitumor | 0.26 |
| 52.187 | Tetracosamethylcyclododecasiloxane | C24O72O12Si12 | 889.8 | Antimicrobial | 0.11 |
| 56.107 | Eicosamethylcyclodecasiloxane | C20H60O10Si10 | 741.84 | Antimicrobial | 0.44 |
Table 6. GCMS analysis of compounds present within the green leaf acetone extracts.
GCMS chemical profiling of the flower extracts
| Retention time | Chart No | Compound name | Molecular formula | Molecular weight | Biological activity | %Area |
|---|---|---|---|---|---|---|
| 10.522 | 1 | O-ethyltoluene | C9H12 | 120.19 | 1.38 | |
| 15.139 | 4 | Undecane | C13H28 | 184.36 | Antimicrobial and antioxidant | 1.2 |
| 12.872 | 5 | Octane, 2,3,6,7-tetramethyl- | C12H26 | 170.33 | 0.3 | |
| 15.9 | 7 | Dodecane | C12H26 | 170.33 | Antioxidant | 0.22 |
| 17.593 | 10 | 1,3-di-tert-butylbenzene | C12H22 | 190.32 | 0.11 | |
| 27.611 | 11 | Nonadecane | C19H40 | 268.5 | 1.57 | |
| 18.873 | 12 | Pentadecane | C15H32 | 212.41 | Antimicrobial and antioxidant | 0.5 |
| 29.389 | 13 | 2,6,10,14-tetramethylhexadecane | C20H42 | 282.5 | 4.01 | |
| 21.657 | 14 | Tetradecane | C14H30 | 198.39 | Antibacterial, diuretic, antituberculosis | 0.32 |
| 23.7 | 18 | 2,6-di-tert-butylquinone | C16H24O | 220.35 | Antidiabetogenic, antioxidant, antiviral, antibacterial, cytotoxic, antifungal | 0.31 |
| 25.017 | 20 | 2,4-di-tert-butylphenol | C14H22O | 206 | Antioxidant, antiviral, antibacterial, cytotoxic, nematoid | 0.45 |
| 27.112 | 22 | 1,2-benzenedicarboxylic acid, dinonyl ester | C12H14O4 | 222.24 | Cytotoxic | 0.28 |
| 30.377 | 24 | Docosane | C22H46 | 310.6 | 1.11 | |
| 49.658 | 29 | Octadecamethylcyclononasiloxane | C18H52O9Si9 | 667.39 | Antimicrobial | 1.74 |
| 46.196 | 30 | Dotriacontane | C32H66 | 450.9 | Antibacterial, antiviral, antifungal, antioxidant, antispasmodic | 3.21 |
| 49.437 | 32 | Octadecane | C18H28 | 254.5 | 2.88 | |
| 34.402 | 33 | 7,9-di-tert-butyl-1-oxaspiro[4.5]deca-6,9-diene-2,8-dione | C17H24O3 | 276.37 | Antimicrobial, antiviral. antineoplastic | 0.9 |
| 39.446 | 40 | 8-N-hexylpentadecane | C21H44 | 296.6 | 2.62 | |
| 43.721 | 45 | 2,2'-methylenebis(4-methyl-6-tert-butylphenol) | C26H34O3 | 394.55 | Antioxidant | 0.73 |
| 48.293 | 46 | Hexatriacontane | C31H64 | 436.84 | Antiinflammatory, antitubercular | 0.28 |
| 52.692 | 51 | Tetrapentacosan $$ tetrapentacontane | C54H110 | 759.4 | Antitumor | 2.87 |
| 51.6 | 55 | Tetracosamethylcyclododecasiloxane | C24H72O12Si12 | 889.8 | Antimicrobial | 2.25 |
Table 7. GCMS analysis of compounds present within the flower petroleum extract.
| Retention time | Compound name | Molecular formula | Molecular weight | Bioactivity | %Area |
|---|---|---|---|---|---|
| 8.002 | 1,1,2,2-tetramethoxy-1,2-dimethyldisilane | C6H18O4Si2 | 210.37 | 0.01 | |
| 8.56 | Dodecane | C12H26 | 170.33 | Antioxidant | 0.02 |
| 8.98 | .Beta.-pinene | C10H16 | 136.23 | Antiinflammatory, antifungal, antiibacterial, antitumor, cytotoxic | 0.05 |
| 9.667 | 1,1,3,3,5,5,7,7-octamethyl-cyclooctasiloxane | C13H39O5Si6 | 443.96 | Antimicrobial | 0.19 |
| 10.291 | 3-tert-butyl-5-chloro-2-hydroxybenzophenone | C17H17CLO2 | 288.8 | Antiarthritic, antiinflammatory, fribinolytic, acute neurologic disorder treatment, fibrinolytic | 0.05 |
| 11.246 | 3-methoxy-4-((trimethylsilyl)oxy)-benzaldehyde-o-methyloxime | C12H19NO3Si | 253.37 | 0.14 | |
| 12.157 | 2,4-dihydroxybenzoic acid methyl ester trimethylsilyl ether | C21H3804Si2 | 410.7 | Antimicrobial | 0.04 |
| 12.951 | 2,3,4-trimethylhexane | C9H20 | 128.25 | 0.04 | |
| 14.439 | Cyclopentasiloxane | C10H30O5Si5 | 370.77 | 0.03 | |
| 17.415 | 1,3-di-tert-butylbenzene | C12H22 | 190.32 | 0.04 | |
| 19.521 | Dodecamethylcyclohexasiloxane | C12H36O6Si6 | 444.92 | Antimicrobial | 0.1 |
| 24.119 | Tetradecamethylcycloheptasiloxane | C14H42Si7O7 | 519.07 | Antimicrobial | 0.09 |
| 24.453 | 2,4-di-tert-butylphenol | C14H22O | 206 | antioxidant, antiviral, antibacterial, cytotoxic, nematoid | 0.09 |
| 25.5 | Cyclopropan, 2-Chloro-1-Ethyl-1-Methyl- | C6H11CL | 118.6 | A potent and selective CRF1 receptor antagonist | 0.05 |
| 26.434 | 1,2-benzenedicarboxylic acid | C12H14O4 | 222.24 | Cytotoxic | 0.17 |
| 28.25 | Hexadecamethylcyclooctasiloxane | C16H48O8Si8 | 593.23 | Antimicrobial | 0.36 |
| 54.585 | Tetracosamethylcyclododecasiloxane | C24O72O12Si12 | 889.8 | Antimicrobial | 0.4 |
| 40.622 | Octadecamethylcyclononasiloxane | C18H54O9Si9 | 667.4 | Antimicrobial | 0.28 |
| 38.64 | Tetradecanoic acid, methyl ester | C15H30O2 | 242.39 | Larvicidal activity and repellant | 0.62 |
| 43.053 | Silikonfett Se30 (grevels) | C6H18OSi2 | 162.379 | Medical lubricant, intraocular tamponade | 0.09 |
| 51.403 | Eicosamethylcyclodecasiloxaneyl- | C20H60O10Si10 | 741.84 | Antimicrobial | 1.23 |
Table 8. GCMS analysis of compounds present within the flower methanol extract.
GCMS chemical profiling of the brown leaf extracts
| Retention time | Compound name | Molecular formula | Molecular weight | Bioactivity | % Area |
|---|---|---|---|---|---|
| 8.941 | O-ethyltoluene | C9H12 | 120.19 | 0.2 | |
| 9.599 | 3,3,5-trimethylheptane | C10H22 | 142 | 0.93 | |
| 10.519 | )2,3-dihydroindene | C18H20 | 236.4 | 0.39 | |
| 27.615 | Decane, 3,7-dimethyl- | C12H28 | 170.33 | 0.58 | |
| 15.901 | Pentadecane | C15H32 | 212.41 | Antimicrobial and antioxidant | 0.26 |
| 17.582 | 2,4-dimethylundecane | C13H28 | 184.36 | Antimicrobial and antioxidant | 0.78 |
| 21.655 | Nonane, 3-methyl-5-propyl | C13H28 | 184.36 | 0.32 | |
| 22.219 | Bicyclo(4.3.0)nonan, 7-methylen-2,4,4-trimethyl-2-vinyl- | C15H24 | 204.36 | 0.29 | |
| 33.356 | Hexadecane, 2,6,10,14-tetramethyl | C20H42 | 282.5 | 10.14 | |
| 25.018 | Tricosane | C23H48 | 324.6 | 0.36 | |
| 26.99 | 1,2-benzenedicarboxylic acid | C12H14O4 | 222.24 | Cytotoxic | 0.22 |
| 28.198 | Tetradecane | C14H30 | 198.39 | Antibacterial, diuretic, antituberculosis | 1.87 |
| 30.379 | Docosane | C22H46 | 310.6 | 0.64 | |
| 31.821 | Nonadecane | C19H40 | 268.5 | 0.14 | |
| 32.44 | Eicosane | C20H42 | 282.5 | Antimicrobial, antiinflammatory | 0.63 |
| 34.06 | Octadecane | C18H28 | 254.5 | 5.13 | |
| 34.931 | Eicosamethylcyclodecasiloxane | C20H60O10Si10 | 741.84 | 0.62 | |
| 36.789 | Hexacosane | C26H54 | 366.71 | Antimicrobial, antiinflammatory | 0.39 |
| 53 | Tetrapentacontan | C54H110 | 759.4 | Antitumor | 2.47 |
| 51.606 | Dotriacontane | C32H66 | 450.9 | Antibacterial, antiviral, antifungal, antioxidant, antispasmodic | 3.48 |
| 41.478 | Tetracosane | C24H50 | 338.7 | Potent inhibitor of b-amyloid aggregation in alzheimer's disease | 0.73 |
| 42.119 | 1,2-Bis(Trimethylsiloxy)-1,2-Diphenylethane | C8H22O2Si2 | 206.43 | 0.52 | |
| 43.059 | C(14a)-homo-27-norgammacer-13-en-21-one, 3-methoxy-, (3.alpha.)- | C31H52O2 | 456.7 | 0.74 | |
| 43.722 | Cinnamyl cinnamate | C18H16O2 | 264.3 | Anticancer, antiinflammatory, antimicrobial, antifungal, anti-hiv, anti tuberculosis | 0.72 |
| 52.233 | Hexacontane | C60H122 | 843.6 | 0.89 | |
| 54.02 | 1,4-epoxynaphthalene-1(2h)-methanol, 4,5,7-tris(1,1-dimethylethyl)-3,4-dihydro- | C11H21NO3 | 215.29 | Antioxidant, cytotoxic | 0.41 |
Table 9. GCMS analysis of compounds present within the brown leaf petroleum ether extract.
| Retention time | Compound name | Molecular formula | Molecular weight | Bioactivity | % area |
|---|---|---|---|---|---|
| 9.662 | 1,1,3,3,5,5,7,7-octamethyl-cyclooctasiloxane | C13H39O5Si6 | 443.96 | Antimicrobial | 0.14 |
| 11.427 | 3-methoxy-4-((trimethylsilyl)oxy)-benzaldehyde-o-methyloxime | C12H19NO3Si | 253.37 | 0.11 | |
| 11.548 | Octane, 3,4,5,6-tetramethyl- | C12H26 | 170.33 | 0.04 | |
| 12.155 | 2,4-dihydroxybenzoic acid methyl ester trimethylsilyl ether | C21H3804Si2 | 410.7 | Antimicrobial | 0.03 |
| 14.438 | Cyclopentasiloxane, decamethyl- | C10H30O5Si5 | 370.77 | Antimicrobial | 0.02 |
| 17.575 | 1,3-di-tert-butylbenzene | C12H22 | 190.32 | 0.03 | |
| 19.507 | Dodecamethylcyclohexasiloxane | C12H36O6Si6 | 444.92 | Antimicrobial | 0.05 |
| 24,118 | Tetradecamethylcycloheptasiloxane | C14H42Si7O7 | 519.07 | Antimicrobial | 0.07 |
| 25.458 | Di-T-butyl-phenol | C14H22O | 206 | antioxidant, antiviral, antibacterial, cytotoxic, nematoid | 0.08 |
| 27.93 | 1,2-benzenedicarboxylic acid | C12H14O4 | 222.24 | Cytotoxic | 0.53 |
| 28.254 | Hexadecamethylcyclooctasiloxane | C16H48O8Si8 | 593.23 | Antimicrobial | 0.16 |
| 28.549 | Pyrazolo(5,1-C)(1,2,4)triazine, 6-(fluoroacetyl)-4,6-dihydro-3-methyl-4-methylene- | Antiviral, antitumor, antimicrobial, antiglycemic, antidepressive, anti-amoebic, antifungal, antioxidant | 0.03 | ||
| 29.81 | 1,1,3,3,5,5,7,7,9,9,11,11-dodecamethyl-hexasiloxane | C16H48O7Si8 | 430.94 | Antimicrobial | 0.21 |
| 31.835 | 1,1,1,5,7,7,7-heptamethyl-3,3-bis(trimethylsiloxy)tetrasiloxane | C13H39O5Si6 | 443.96 | 0.02 | |
| 38.681 | Octadecanoic acid | C19H38O2 | 298.5 | Cancer preventive | 0.4 |
| 41.836 | Tetracosamethylcyclododecasiloxane | C24O72O12Si12 | 889.8 | Antimicrobial | 0.29 |
| 49.675 | Octadecamethylcyclononasiloxane | C18H54O9Si9 | 667.4 | Antimicrobial | 1.02 |
Table 10. GCMS analysis of compounds present within the brown leaf methanol.
| Retention time | Compound name | Molecular formula | Molecular weight | Bioactivity | % area |
|---|---|---|---|---|---|
| 8.059 | Trifluormethyl-trimethylsilan | C4H8F3Si | 142.19 | 0.62 | |
| 10.077 | 2,2,3,3-tetramethylhexane | C9H20 | 128.25 | 0.26 | |
| 10.343 | 3,3,5-trimethylheptane | C10H22 | 142 | 0.4 | |
| 11.459 | Undecane, 2,7-dimethyl | C13H26 | 184.36 | Antimicrobial and antioxidant | 0.6 |
| 12.869 | Decane, 3,7-dimethyl | C11H24 | 156.31 | 0.33 | |
| 16.023 | Pentadecane | C15H32 | 212.41 | Antimicrobial and antioxidant | 0.19 |
| 17.614 | 1,3-di-tert-butylbenzene | C12H22 | 190.32 | Pharmaceutical intermediate | 0.13 |
| 18.252 | Nonadecane | C19H40 | 268.5 | 0.55 | |
| 19.079 | Tetradecane, 5-methyl | C14H30 | 198.39 | Antibacterial, diuretic, antituberculosis | 0.3 |
| 19.575 | 2,6,10,14-tetramethyl | C20H42 | 282.5 | 0.41 | |
| 25.034 | 2,4-di-tert-butylphenol | C14H22O | 206 | Antioxidant, antiviral, antibacterial, cytotoxic, nematoid | 0.72 |
| 26.355 | 2,6-di-tert-butyl-4-ethylphenol | C16H26O | 234.38 | 0.35 | |
| 27.61 | Nonadecane | C19H40 | 268.5 | 0.68 | |
| 30.376 | Hexadecane, 2,6,10,14-tetramethyl- | C19H40 | 268.5 | 2.32 | |
| 32.441 | Eicosane | C20H42 | 282.5 | Antimicrobial, antiinflammatory | 0.51 |
| 34.43 | 7,9-di-tert-butyl-1-oxaspiro[4.5]deca-6,9-diene-2,8-dione | C17H24O3 | 276.37 | antimicrobial, antiviral, antineoplastic | 0.3 |
| 35.422 | Hexadecanoic acid | C6H32O2 | 256.42 | Antioxidant, antimicrobial, antiinflammatory hypocholesterolemic | 0.81 |
| 38.248 | Tetracosane, 2,6,10,15,19,23-hexamethyl- | C30H62 | 422.8 | 0.11 | |
| 39.437 | Octadecanoic acid | C19H38O2 | 298.5 | Cancer preventive | 0.93 |
| 41.574 | Tetracosane | C24H50 | 338.65 | Potent inhibitor of B-amyloid aggregation in Alzheimer's disease | 0.3 |
| 44.903 | Dotriacontane | C32H66 | 450.9 | Antibacterial, antiviral, antifungal, antioxidant, antispasmodic | 3.74 |
| 45.507 | Octadecane | C18H28 | 254.5 | 1.12 | |
| 47.599 | 1,3,5-trisilacyclohexane | C6H6Si3 | 126.33 | 0.2 | |
| 49.579 | Acetic acid 13-hydroxy-4,4,6a,6b,8a,11,11,14b-octamethyl-docosahydro-picen-3-yl | C32H54O3 | 486.8 | 0.71 | |
| 50.291 | Lupan-3.beta.-ol (triterpenoids) | C30H52O | 428.7 | Antiinflammatory, antiprotozoal, antimicrobial, antitumor, chemo preventive | 0.11 |
| 52.677 | Tetrapentacosan | C54H110 | 759.4 | Antitumor | 1.85 |
| 54.091 | 1,4-epoxynaphthalene-1(2h)-methanol, 4,5,7-tris(1,1-dimethylethyl)-3,4-dihydro- | C11H21NO3 | 215.29 | Antioxidant, cytotoxic | 0.23 |
| 55.712 | Tetracontane | C40H82 | 563.1 | Antibacterial | 1.07 |
Table 11. GCMS analysis of compounds present within the brown leaf ethyl ether.
| Retention time | Compound name | Molecular formula | Molecular weight | Bioactivity | % Area |
|---|---|---|---|---|---|
| 7.536 | Isopropylbenzene | C9H12 | 120.19 | Antioxidant, antinociceptive, anxiolytic, anticancer, antinociceptive | 0.13 |
| 8.559 | 2-methylnonane | C10H22 | 142.28 | 0.05 | |
| 8.923 | 2,5-dimethylnonane | C11H24 | 156.31 | 0.1 | |
| 9.135 | N,n,n',n'-tetramethyldiaminomethane | C5H14N2 | 102.18 | 0 | |
| 9.299 | 6-hydroxynobiline | C17H27NO4 | 309.4 | Neuroprotective, antitumor, liver homeostasis | 0 |
| 9.467 | Pseudoephedrine | C10H15NO | 165.23 | Decongestant | 0.01 |
| 9.667 | 2-n-hexyl-1-d1-aziridine | CH2NHCH2 | 43.07 | Scaffold for bioactive compounds-Pyrazinoindole derivatives | 0.1 |
| 10.359 | 3,3,5-trimethylheptane | C10H22 | 142 | 0.21 | |
| 11.461 | Undecane, 2,7-dimethyl | C13H26 | 184.36 | Antimicrobial and Antioxidant | 0.28 |
| 14.191 | Conessin (steroidal alkaloid) | C24H40N2 | 356.6 | Antidiarrheal, antibacterial resistance modifying agent, antimalarial, h3 receptor antagonist | 0 |
| 14.384 | Cyclopentasiloxane, decamethyl- | C10H30O5Si5 | 370.77 | Antimicrobial | 0.04 |
| 19.451 | Dodecamethylcyclohexasiloxane | C12H36O6Si6 | 444.92 | Antimicrobial | 0.13 |
| 20.05 | N-methylpiperidine | C6H13N | 99.17 | 0.01 | |
| 23.983 | Lucenin 2(flavone glycoside) | C21H20O11 | 448.4 | Antibacterial, antitumor, antiinflammatory, antioxidant, antihepatotoxic | 0.03 |
| 25.469 | Pentansaeure, 5-hydroxy-, (2,4-ditert.butylphenyl)ester | C19H30O3 | 306.4 | Neuroprotective | 0.35 |
| 27.899 | 1,2-Benzenedicarboxylic acid, dibutyl ester | C12H14O4 | 222.24 | Cytotoxic | 0.27 |
| 28.243 | Hexadecamethylcyclooctasiloxane | C16H48O8Si8 | 593.23 | Antimicrobial | 0.59 |
| 30.548 | Hexadecane, 2,6,10,14-tetramethyl | C19H40 | 268.5 | 0.86 | |
| 31.821 | Silikonfett SE30 (GREVELS) $$ Silicone oil | C6H18OSi2 | 162.379 | Medical lubricant, intraocular tamponade | 0.09 |
| 33.901 | Glycerol | C53H98O6 | 831.4 | Laxative, antimicrobial, antiviral | 0.05 |
| 35.176 | Docosane | C22H46 | 310.6 | 0.08 | |
| 38.348 | Tetrapentacosan $$ tetrapentacontane | C54H110 | 759.4 | Antitumor | 0.17 |
| 39.125 | Octadecane | C18H28 | 254.5 | 0.17 | |
| 43.042 | Octadecamethylcyclononasiloxane | C18H54O9Si9 | 667.4 | Antimicrobial | 0.18 |
| 43.868 | 2,2'-methylenebis(4-methyl-6-tert-butylphenol) | C26H34O3 | 394.55 | Antioxidant | 0.16 |
| 48.798 | 3-ethyl-5-(2'-ethylbutyl)octadecane | C26H54 | 366.7 | Antimicrobial and antifungal | 0.16 |
| 55.943 | Dotriacontane | C32H66 | 450.9 | Antibacterial, antiviral, antifungal, antioxidant, antispasmodic | 0.73 |
Table 12. GCMS analysis of compounds present within the brown leaf acetone extract and their bioactivity.
| No | Bioactive compound | Nature | Molecular formula | Extract |
|---|---|---|---|---|
| 1 | 2,6-di-tert-Butylquinone | Monoterpenoid | 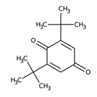 |
GL/PE, F/PE |
| 2 | Tetrapentacosan $$ Tetrapentacontane | Alkane |  |
GL/PE, GL/EE, GL/A, F/PE, BL/PE, BL/EE, BL/A |
| 3 | Decamethylcyclopentasiloxane | terpenoid | 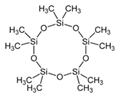 |
GL/PE, GL/MeOH, GL/A, F/PE, F/MeOH, BL/MeOH, BL/A |
| 4 | 2,2'-Methylenebis(4-methyl-6-tert-butylphenol) | Phenol | 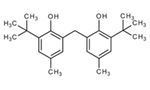 |
GL/PE, GL/EE, GL/A, F/PE, BL.A |
| 5 | 3-Ethyl-5-(2'-ethylbutyl)Octadecane | Long chain alkane | 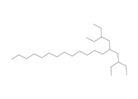 |
BL/A |
| 6 | Dotriacontane | Alkane |  |
GL/PE, GL/EE, GL/A, F/PE, BL/PE, BL.EE, BL/A |
| 7 | SILIKONFETT SE30 (GREVELS) $$ Silicone oil | lipids | 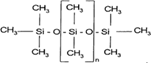 |
F/MeOH, BL/A |
| 8 | Glycerol | lipid |  |
BL.A |
| 9 | 6-hydroxynobiline | Alkaloid | 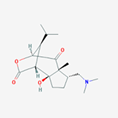 |
BL/A |
| 10 | Pseudoephedrine | Alkaloid | 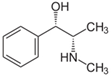 |
GL/A, BL/A |
| 11 | 2-n-hexyl-1-d1-aziridine | Alkaloid | 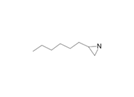 |
GL/A, BL.A |
| 12 | Conessin | Steroid alkaloid | 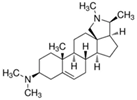 |
BL/A |
| 13 | Cyclopentasiloxane, decamethyl- | terpenoid | 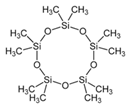 |
GL/MeOH, GL/A, BL/MeOH, BL/A |
| 14 | Dodecamethylcyclohexasiloxane | terpenoid | 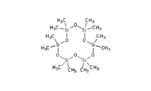 |
GL/M, GL/A, F/MeOH, BL/MeOH, BL/A |
| 15 | Lucenin 2 | Flavonoid C-glucosyl | 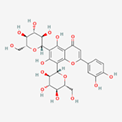 |
BL/A |
| 16 | Pentansaeure, 5-hydroxy-, (2,4-ditert.butylphenyl)ester | Phenol ester | 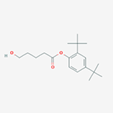 |
BL/A |
| 17 | 2,4 Dihydrobenzoic acid | Phenolic acid | 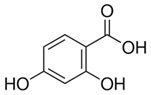 |
GL/MeOH, F/MeOH, BL/MeOH |
| 18 | Hexadecamethylcyclooctasiloxane | terpenoids | 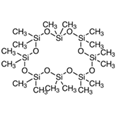 |
GL/MeOH, F/MeOH, BL/MeOH, BL/A |
| 19 | 2,6-Di-Tert-Butyl-4-Ethylphenol | phenol | 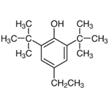 |
BL/EE |
| 20 | Octadecanoic Acid | Fatty acid | 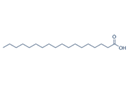 |
GL/MeOH, BL/MeOH, BL/EE, |
| 21 | Tetracosamethylcyclododecasiloxane | terpenoid | 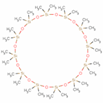 |
GL/PE, GL/M, GL/A, F/PE. F/MeOH, BL/MeOH |
| 22 | 7,9-Di-Tert-Butyl-1-Oxaspiro[4.5]Deca-6,9-Diene-2,8-Dione | Flavonoid | 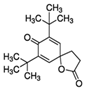 |
GL/PE, GL/EE, GL/A, F/PE, BL/EE |
| 23 | Hexadecanoic Acid | Saturated fatty acid | 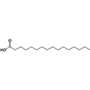 |
BL/EE |
| 24 | Tetradecamethylcycloheptasiloxane | terpenoid | 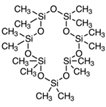 |
GL/MeOH, GL/A,F/MeOH, BL/MeOH |
| 25 | Di-T-Butyl-Phenol | Flavonoid | 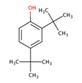 |
GL/PE, GL/EE, F/PE, F/MeOH, BL/MeOH, BL/EE, |
| 26 | Pyrazolo[5,1-C][1,2,4]Triazine, 6-(Fluoroacetyl)-4,6-Dihydro-3-Methyl-4-Methylene- | Alkaloid | 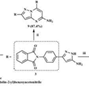 |
BL/MeOH, |
| 27 | 3,5-Di-Tert-Butylphenol | Alkylated phenol | 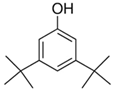 |
GL/MeOH |
| 28 | Caryophyllene Oxide | Sesquiterpenoid |  |
GL/MeOH, |
| 29 | Eicosamethylcyclodecasiloxane | Terpenoid |  |
GL/MeOH, GL/A, F/M, BL/PE, |
| 30 | Lupan-3.Beta.-Ol (Triterpenoids) | Triterpenoid |  |
BL/EE |
| 31 | 1,4-Epoxynaphthalene-1(2h)-Methanol, 4,5,7-Tris(1,1-Dimethylethyl)-3,4-Dihydro- | Alkaloid |  |
BL/PE, BL/EE |
| 32 | 4-T-Butoxy-1-Methyl-1,2,3,6,-Tetrahydropyridine | Alkaloid | 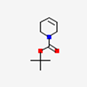 |
GL/A |
| 33 | Eicosane | Alkane | 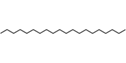 |
BL/PE, BL/EE, |
| 34 | Hexacosane | Alkanes | 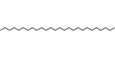 |
BL/PE |
| 35 | Hentriacontane | Alkane |  |
GL/PE |
| 36 | 1,3-Dioxolane, 2-(Phenylmethyl) | Terpenoid | 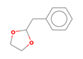 |
GL/EE |
| 37 | 2,7-Dimethyloctane | Alkane | 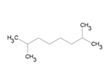 |
GL/A |
| 38 | Tetracontane | Alkane |  |
BL/EE |
| 39 | Cinnamyl Cinnamate | Fatty acid | 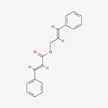 |
BL/PE |
| 40 | Dodecane | Alkane | 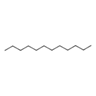 |
GL/PE, GL/EE, F/PE, F/MeOH, |
| 41 | Tetradecane | Alkane | 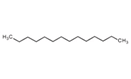 |
GL/PE, GL/EE, F/PE, BL/PE, BL/EE |
| 42 | Pentadecane | Alkane | 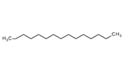 |
GL/PE, F/PE, BL/PE, BL/EE |
| 43 | Undecane | Alkane | 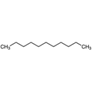 |
GL/PE, GL/EE, GL/A, F/PE, BL/PE, BL/EE, BL/A |
| 44 | 1,2 Benzencarboxylic acid | Phenolic acid | 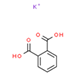 |
GL/MeOH, GL/EE, GL/A, F/PE, F/MeOH, BL/PE, BL/MeOH, BL/A |
| 45 | Isopropyl benzene | Monoterpenoid | 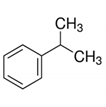 |
GL/A, BL/A |
| 46 | Alpha-Pinene | Monoterpenoid | 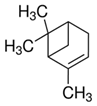 |
GL/MeOH |
| 47 | 3-Tert-Butyl-5-Chloro-2-Hydroxybenzophenone | Phenolic | 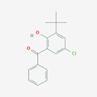 |
F/MeOH |
| 48 | Beta-pinene | Monoterpenoid | 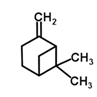 |
GL/M, F/MeOH |
| 49 | Cyclopropan, 2-Chloro-1-Ethyl-1-Methyl- | Alkaloid |  |
F/M |
| 50 | Octadecamethylcyclononasiloxane | Terpenoids | 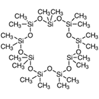 |
GL/M, GL/PE, GL/A, F/PE, F/MeOH, BL/MeOH, BL/A |
| 51 | Tetradecanoic Acid, Methyl Ester | Saturated fatty acids | 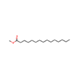 |
F/MeOH |
| 52 | Tetracosane | Alkane |  |
GL/EE,BL/PEBL/EE, |
Table 13. The nature of various bioactive phytoconstituents present in all extracts.
Sideritis cypria is an endangered endemic species in Cyprus. It is a small shrub measuring 34.5 cm in height. The distribution of Sideritis cypria in North Cyprus is sparse because of its preferred habitat on rocky soil between the rock clefts or dry limestone cliffs facing South, under sufficient exposure to sunlight. For decades, the plant has been used to treat various inflammatory and infectious conditions. Being a reported traditional herbal drug, recent studies have been conducted to reassert the biological properties of S.cypria.
As evidenced by the preliminary phytochemical screening, all aerial parts of the wild plant reveal the presence of phenols, triterpenoids, sesquiterpenoids, fatty acids, steroids, and alkaloids. These compounds likely contribute to the high antimicrobial activity of the plant. The result of subsequent antimicrobial analysis test affirms the bioactive properties of s.cypria. The results showed substantial antibacterial activity of all the extracts especially the brown leaf-acetone and the flower petroleum ether extract. When compared to ciprofloxacin, the positive control, aerial parts of S.cypria displayed a wide range of antimicrobial activity against both gram-positive and gram-negative bacteria lesser than that of ciprofloxacin at a certain concentration. A better study focusing on the concentration of the extracts will make a better comparison. The extracts from the flower displayed a wide range of bioactivity both in the antimicrobial analysis and with the chemical profiling results. This is not a novel finding as previous literature by Lytra, et al., on the phytochemistry, nutritional content, and bioactivity of Sideritis cypria reported higher bioactivity of the components of the flower than that in the leaves. For this study, this might have been the case if there was a flower/acetone extract to be compared with the brown leaf/acetone extract which had the highest yield and the highest antimicrobial activity. Unfortunately, the only extracts prepared from the flower were with methanol and petroleum ether due to sparse resources. The antimicrobial property of the brown leaf was shown to be higher than that of the green leaf seeing that both the acetone and methanol extract of the brown leaf exhibited the widest zones of microbial inhibition repeatedly, which makes it probable that the brown leaf of s.cypria contains higher levels of potent antimicrobial phytoconstituents than the other parts of the plant studied. This is also reaffirmed by the GCMS profile results.
This study successfully isolated 52 bioactive phytoconstituents from 8 different extracts of the aerial parts of wild s.cypria which include 15 terpenoids (majorly sesquiterpenoids and monoterpenoids), 14 alkanes, 7 alkaloids, 7 phenols, 4 fatty acids, 2 flavonoids, and 1 steroid. Some of the most common bioactive phytoconstituents found in almost all the plant materials include 1,2 benzene dicarboxylic acid, organosilanes, Tetrapentacontane, dotriacontane, di tert-butyl pheno, Oxaspiro (4.5)Deca-6,9-Diene-2,8-Dione, and Undecane which have known antimicrobial, antioxidant, and other bioactive properties. However, some less occuring, yet unique bioactive compounds were also isolated such as Pseudoephedrine, Conessin, Lecenin2, and Lupan-3.Beta.-Ol, 6-hydroxynobiline, Aziridine, and Cinnamyl Cinnamate. The high levels of mono and sesquiterpenoids also reported in other Sideritis species is the probable cause of the high antimicrobial activity of s.cypria. The consistently high %area of Dotriacontane in many of the extracts especially in the brown leaf extracts may also suggest a relationship between the bioactivity of this plant and presence of the compound. However, the extract with the highest antimicrobial activity-BL/A, showed not as much high levels (0.73%) of Dotriacontane as with the other extracts (>3%).
The quantity and type of phytoconstituents as shown in the results vary according to the part of the plant, and according to the type of solvent used for extraction. Generally, acetone extracts were observed to have yielded compounds which were not present in any of the other extracts. Among all extracts, the brown leaf acetone extract had the highest yield of bioactive phytoconstituents. The acetone extract of the green leaf also displayed a wide variety of bioactive phytoconstituents but not as much as that of the brown leaf. Therefore, in bioactivity screening of the S.cypria, it would be safe to deduce that acetone is the preferred solvent of extraction, and the plant material with the highest bioactivity is the brown leaf and the flower. Some bioactive phytoconstituents isolated exhibit a wide range of bioactivity not only excluded to antimicrobial activity, but also have antioxidant, anti-HIV, cytotoxic, anticancer, decongestant, anti-amyloid aggregation, antidiabetogenic, antidepressant, antitubercular properties. This will guide better and advanced use of the crude drug extract of the S.cypria. The rich molecular composition of under studied sideritis cypria endemic to cyprus holds great potential for developing future therapeutic candidates.
Authors have no conflict of interest.
The study was conceived and carried out by Judith Uchechi Anyaba, Ovgu Isbilen. Plants were collected by Ender Volkan, Ovgu Isbilen, and Judith Uchechi Anyaba. Preparation and all experiments were done by Judith Uchechi Anyaba. The pictures, design and written document was done by Judith Uchechi Anyaba. The editing was done by Judith Uchechi Anyaba. All authprs have read and approved the paper.
The greatest accolade for this research goes to god, and to the entire staff of the faculty of pharmacy, Cyprus international university for their various contributions during the experimental process of this research.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]