ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Venugopal Gopikrishnan1, Raasaiyah Pazhanimurugan1, Thangavel Shanmugasundaram1, Manikkam Radhakrishnan2 and Ramasamy Balagurunathan1*
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The problems associated with environmental biofouling and the limitation of currently available antifouling compounds has intensified the search for novel eco-friendly antifouling compounds from new sources. The present study reports bioprospecting of culturable actinobacteria from less explored marine ecosystems against biofouling bacteria. Fifty five bacterial isolates were recovered from fouling samples collected from Muttom, Parangipettai, Nagapattinam and Ennore coastal areas. Morphologically distinct isolates were phenotypically characterized. The isolates were belongs to species of the genus Bacillus, Aeromonas, Micrococcus, Alcaligenes, Lactobacillus, Staphylococcus, Pseudomonas and Kurthia. Biofilm forming capacity of all the isolates evaluated by adopting plate as well as tube method. Out of 55 bacterial isolates, 25 isolates produced positive results for biofilm formation in which the species belongs to the genus Staphylococcus, Micrococcus, Vibrio and Alcaligenes were identified as strong biofilm producers. For the isolation of potential antifouling compounds, totally 50 actinobacterial isolates were recovered from mangrove and estuarine sediment samples collected from Parangipettai and Pitchavaram coastal areas, Tamil Nadu and were phenotypically characterized. In agar plug method, 42 out of 50 actinobacterial isolates inhibited one/more number of biofouling bacteria tested. Two actinobacterial isolates viz., strain PM33 and the strain PE7 showed promising activity against maximum number of biofouling bacteria tested. Isolation of bioactive metabolites from both the actinobacterial strains will leads to the development of promising antifouling candidates
Keywords |
| Biofouling bacteria, mangrove, estuary, biofilm, actinobacteria, antifouling compounds. |
INTRODUCTION |
| Marine biofouling is a major talkative phenomenon related with far-reaching consequences leads to larger economic loss and operational failure in maritime and aquaculture industries (Kwong et al., 2006). In the marine environment, natural and artificial surfaces, when contact with the marine water, are quickly colonized by microfoulers like bacteria, algae, protozoa and macrofoulers like barnacles, bryozoans and tubeworms (Callow and Callow, 2002; Dobretsov et al., 2006). These fouling organisms creates huge economic and material loss by settling on man-made surfaces such as ship hulls, cooling system pipes of power stations and mariculture facilities. This leads to decreasing of ship speed and maneuverability, increasing of fuel and cleaning expenses (Lewis, 1994). In the case of shellfish aquaculture, economic losses resulting from over-settlement and smoothening of the crop (Hecht and Heasman, 1999; Carver et al., 2003; Lane and Willemsen, 2004). In order to prevent the marine biofouling, broad spectrum metal biocides, such as tributyl tin (TBT) and copper have been used as antifouling compounds (Albert et al., 1992; Thomas et al., 2001). These biocides were very effective, but highly toxic to non-target organisms (Alzieu, 2000; Konstantinou and Albanis, 2004). Due to this adverse effect, International Maritime Organization (IMO) and Marine Environmental Protection Committee (MEPC) banned the usage of TBT or other substances containing tin as biocides in antifouling paints from January 2008 (Xu et al., 2010). Therefore it is necessary to develop alternative antifoulants that are environmental friendly, as well as economically viable for maritime domains. Actinobacteria are the promising group of eubacteria in terms of biodiversity and bioactive metabolite production in particular antibiotics. According to the recent report by Berdy (2012), so far around 13,700 microbial metabolites are reported from the members of the group actinobacteria. In the recent years marine derived actinobacteria are under exploration for novel bioactive metabolites including antifouling compounds (Lam, 2006). In India, research on marine actinobacteria with special reference to antifouling compounds is still in the stage of infancy. The present study has been attempted for the isolation and characterization of biofouling bacteria from marine samples and also reports the antagonistic actinobacteria from selected mangrove and estuarine sediments of South East Coast of Tamil Nadu with special reference to antifouling activity. |
II. MATERIALS AND METHODS |
| A. Collection of biofouling samples |
| Biofouling samples were scrapped from the boat surfaces and other marine structures around Muttom (M), Kanyakumari District (Lat. 8017âÃâ¬ÃŸ96” N; Long. 77044âÃâ¬ÃŸ74” E), Parangipettai (P) (Lat. 11030âÃâ¬ÃŸ 33” N; Long. 79043âÃâ¬ÃŸ 13âÃâ¬ÃŸÃ¢Ãâ¬ÃŸ E), Nagapattinam (N) (Lat. 10046âÃâ¬ÃŸ0” N; Long. 79050âÃâ¬ÃŸ0” E) and Ennore (E) (Lat. 13021âÃâ¬ÃŸ75” N; Long. 80032âÃâ¬ÃŸ16” E) coastal areas, Tamil Nadu. All the samples were collected in sterile polythene bags and aseptically transferred into the laboratory. |
| B. Isolation and characterization of biofouling bacteria |
| About one gram of fouling sample was serially diluted using sterile distilled water blanks. One hundred microlitre of aliquot from 10-3 to 10-5 was transferred to nutrient agar plates prepared in 50% filtered sea water and spreaded using sterile L-rod. Plating was done in duplicate and all the plates were incubated at 280C for 3-5 days. After enumeration, morphologically different bacterial colonies were selected, purified and sub-cultured on nutrient agar slants supplemented with 2% NaCl (Bavya et al., 2011). |
| C. Biofilm formation study on isolated bacteria |
| 1. Plate Method: |
| Mucoid nature of the bacterial colonies was studied by cultivation of all the strains on congo red agar (CRA) plates (Mariana et al., 2009). Eighteen hours old bacterial cultures were taken and spotted on the CRA plates. Plates were incubated at 28°C for 24-48 hours and observed for the characteristic colony morphology. |
| 2. Tube method: |
| The capability of biofilm formation by the bacterial isolates was noticed by the way of adherence to the walls of culture tubes (Mathur et al., 2006). Inoculum was prepared using 2 ml of nutrient broth. After 24 hours of incubation at 280C, turbidity was adjusted to 0.5 McFarland standards. For biofilm experiment, 100μl of inoculum was transferred into 3ml of nutrient broth in 10 ml test tubes. All the test tubes were kept in shaker at 95 RPM speed for 24-48 hours. After incubation, culture broth which contains the free cells, if any, were discarded. The tubes were washed with 3ml of 1X phosphate buffer saline (PBS). About 3ml of 2 % crystal violet solution was added and allowed to act for 5 minutes. All the tubes were washed with sterile water after discarding the crystal violet solution and allowed to dry. All the tubes were visually observed for the presence of biofilms on the inner walls of the test tubes. All the tubes were added with 1.5ml of 33 % glacial acetic acid and mixed gently. Optical density (OD) value was measured in colorimeter at 570 nm. The OD values of the test samples were compared with the PBS present in the control tube. |
| D. Characterization and identification of biofouling bacteria |
| Phenotypic characteristics such as micro morphology (gram staining, capsule staining, endospore staining and motility), cultural characteristics (on basal-, differential- and selective-media) and biochemical characteristics of all the bacterial isolates were studied by adopting standard procedures (Cappuccino and Sherman, 2005). Effect of sea water on growth of biofilm forming bacterial isolates were also studied using nutrient agar supplemented with different concentration of sea water. Based on phenotypic characteristics, the selected biofouling bacteria were identified at genus level. |
| E. Isolation of marine actinobacteria |
| Sediment samples were collected from Parangipettai (Lat. 11030âÃâ¬ÃŸ33” N; Long. 79043âÃâ¬ÃŸ13âÃâ¬ÃŸÃ¢Ãâ¬ÃŸ E) mangrove rhizosphere region (PM), Parangipettai estuary (PE) and Pitchavaram (P) (Lat. 11° 39' 0" N; Long. 79066âÃâ¬ÃŸ62” E) coastal areas, Tamil Nadu. The central portions of sediment samples were collected, after removing the surface layer and transferred in to sterile plastic bag. The collected sediment samples were air dried at room temperature for a week and it was transferred to sterile petriplates and kept at 55°C for 10 minutes in order to retard the growth of slime forming bacteria (Pisano et al., 1986). Ten grams of dried sediment samples were added in to 90 ml of sterile distilled water and serially diluted up to 10-5 dilutions. Starch casein agar (SCA) medium was prepared in 50% sea water and used for the isolation of culturable actinobacteria. The SCA medium was supplemented with nalidixic acid (20 ïÃÂÃÂg/ml) and cycloheximide (50 ïÃÂÃÂg/ml) to retard the growth of bacteria other than actinobacteria and fungi, respectively. About 0.1 ml of aliquot from each dilution was transferred into SCA plates and spreaded using sterile L rod. All the plates were incubated at 28±2ïÃâðC, and observed from 5th day onwards for one month. The same procedure was followed for all the samples. Colonies with suspected actinobacteria morphology were enumerated and purified using yeast extract-malt extract agar medium (Shirling and Gottileb1966). The pure cultures of the actinobacteria were maintained as slant stock on ISP2 agar as well as in 30% glycerol broth at 40C. All the media used in this study were prepared in 50% filtered sea water unless otherwise stated (Bavya et al., 2011). |
| F. Characterization and dereplication of actinobacteria: |
| Cultural characterization was done by inoculating all the actinobacterial cultures into ISP2 agar medium. All the plates were incubated for 10 days at 280C. Cultural characteristics recorded include growth, consistency, aerial mass colour, presence of reverse side pigment and soluble pigment production (Shirling and Gottileb, 1966). Micromorphological characteristics were studied by adopting slide culture method (Radhakrishnan et al., 2011). About 2 ml of ISP2 agar medium inoculated with actinomycete spores were poured as a thin layer over the surface of sterile microscopic slides. The slides were kept in sterile petriplates and incubated at 280C for 10 days. Then the slides were observed under bright field microscope at 40X magnification. The recorded microscopic characteristics include presence of aerial mycelium, substrate mycelium, mycelial fragmentation and spore chain morphology. Based on the results of growth pattern of actinobacteria on ISP2 agar medium and microscopic appearance, similar actinomycete isolates were discarded and different isolates were selected for further investigations. The selected isolates were grouped into Streptomycetes and non-Streptomycetes / rare actinobacteria. |
| G. Screening of actinobacteria against biofouling bacteria |
| All the actinobacterial isolates were screened for their antagonistic activity against biofouling bacteria by agar plug method. Actinomycete cultures were inoculated into YEME agar plates (20 ml/plate) and incubated at 280C for 10 days. After scraping the mycelial growth, 5 mm diameter agar plug was cut from YEME agar plates. Test organisms were inoculated into nutrient agar plates using sterile cotton swab. The agar plugs were placed over nutrient agar seeded with test organisms. All the plates were incubated at 280C for 24 hours. Zone of inhibition was expressed in millimetre in diameter (Eccleston et al., 2008). Actinobacterial isolates showing maximum zone of inhibition against wide range of biofouling bacteria were selected as potential candidates for the further isolation of antifouling compounds. |
| H. Production of antifouling metabolite from selected isolates |
| Effect of solid and submerged culture on antifouling metabolite production by the strain PE7 and PM33 was investigated. Both the strains were inoculated into YEME agar plates as well as in each 100 ml of YEME broth. Both the medium were prepared in distilled water as well as in sea water. YEME agar plates were incubated at 280C for 12 days. YEME broth containing flasks were incubated in rotary shaker with 95 RPM for 12 days. For every 24 hours, agar plug from YEME agar plates were taken and tested against biofouling bacteria (Eccleston et al. 2008). Each 2 ml of YEME broth from all the flasks were taken and centrifuged at 10000 RPM for 10 minutes. The cell free supernatant was tested against the biofouling bacteria by adopting well diffusion method (Bavya et al., 2011). |
III. RESULTS |
| A. Culturable bacteria from biofouling samples |
| All the collected biofouling samples are slimy in nature with brown or green colour appearance. Total culturable bacterial population present in the biofouling samples collected from different coastal area were estimated (Table 1). |
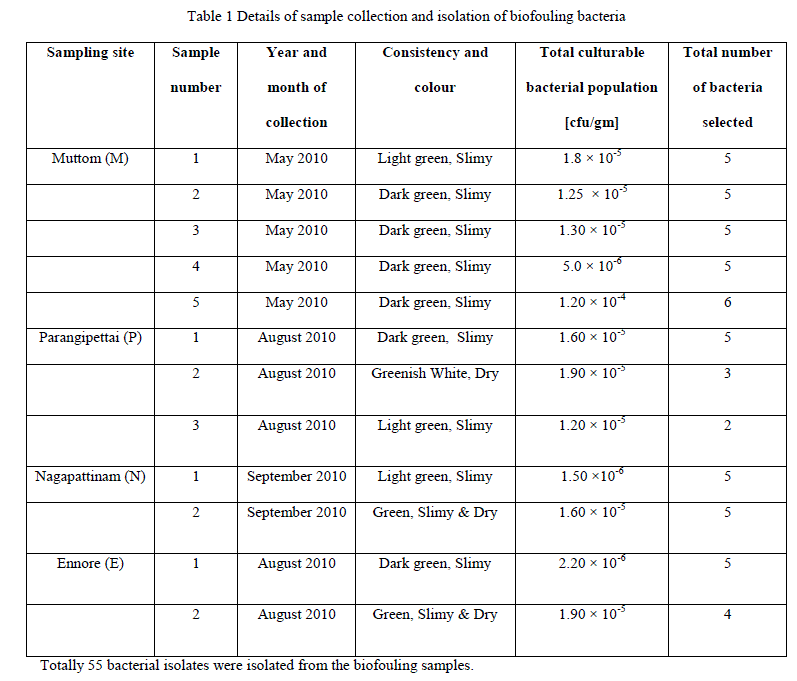 |
| B. Characterization and identification of biofouling bacteria |
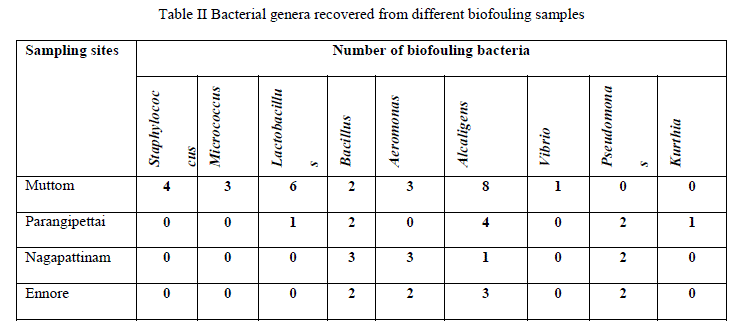
| All the 55 bacterial isolates were identified at genus level. Bacteria isolated from different biofouling samples belongs to the genus Bacillus sp. (M38, M56, P5, P13, N2, N12, N16, E15, E29), Aeromonas sp. (M13, M19, M20, N5, N6, N8, E6, E8), Micrococcus sp (M11, M23, M50), Alcaligenes sp. (M2, M28, M30, M33, M47, M48, M52, M54, P2, P8, P9, P14, N15, E4, E22, E26), Lactobacillus sp. (M6, M14, M21, M46, M51,M55,P4), and Staphylococcus sp. (M1, M3, M27, M45), Pseudomonas sp. (P1, P11, N9, N13, E2, E28), Vibrio sp. (M25), Kurthia sp. (P3). Number of bacterial isolates recovered from different biofouling samples were given in table 2. |
 |
| Viability of all the isolates was maintained as slant culture and stab culture on nutrient agar slants. Effect of seawater showed that most of the bacterial isolates showed poor growth on medium prepared without sea water. Certain biofouling bacterial isolates viz., M1, M11, P13 and N9 produced poor growth and failed to produce pigmentation on the medium prepared without sea water. |
| C. Confirmation for biofilm formation: |
| 1. Plate Method: |
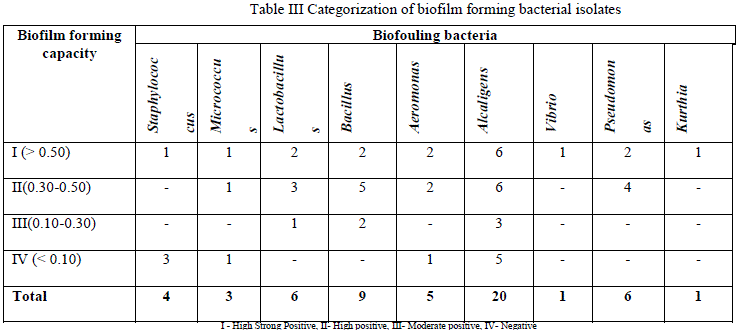
| Out of 55 bacterial isolates, 25 isolates produced positive results for biofilm formation in plate method. Among the various genera isolated Staphylococcus, Micrococcus, Vibrio and Alcaligenes genera showed strong positive results. |
| 2. Tube method: |
| Out of 55 bacterial isolates, 25 isolates produced positive results for biofilm formation in tube method. In tube method also, bacterial genera such as Staphylococcus, Micrococcus, Vibrio and Alcaligenes showed strong positive result for biofilm formation (Table 3). |
 |
| D. Isolation and characterization of actinobacteria |
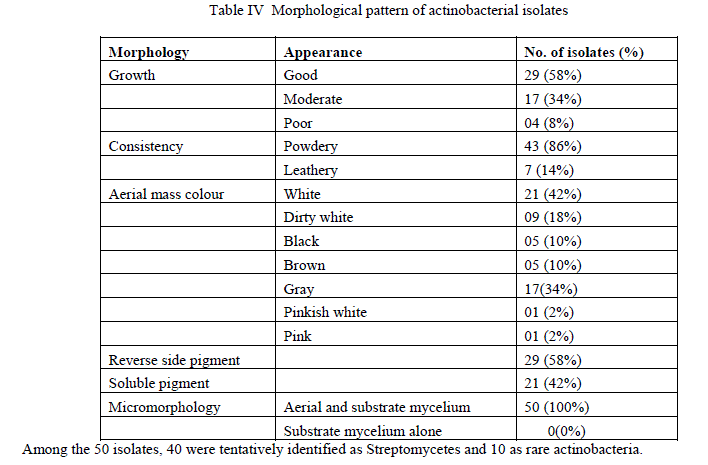
| Culturable actinobacterial population in Pitchavaram ecosystem was estimated as 3.2 X 104 cfu/gm, in Parangipettai mangrove rhizosphere 7.2 X 105 cfu/gm and in Parangipettai estuarine region 2.5 X 104 cfu/gm of sediment. Totally 50 actinobacteria, each 20 from Pitchavaram and Parangipettai mangrove rhizosphere and 10 isolates from Parangipettai estuarine region were selected for further antagonistic studies.About 58% of the actinobacterial isolates produced good growth on ISP2 agar medium. Forty two percent of the isolates produced white aerial mycelium followed by 34% of the isolates produced gray colour aerial mycelium. Both aerial and substrate mycelium was produced by all the actinobacterial isolates (Table 4). |
 |
| E. Antagonistic activity of actinobacteria against Biofouling bacteria |
| Of the 50 actinobacterial isolates screened, when compared to Pitchavaram ecosystem, actinobacteria from Parangipettai mangrove and estuarine ecosystems were showed promising activity against the biofouling bacteria tested (Fig 1). |
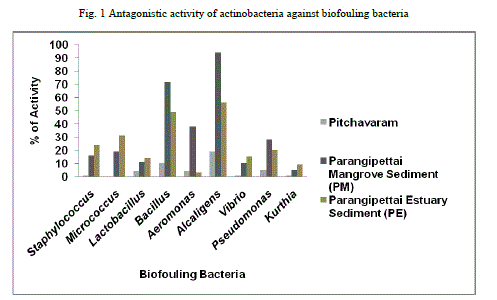 |
| Actinobacterial strains PM33 and PE7 showed good inhibition against maximum number of biofouling bacteria (Table 5). |
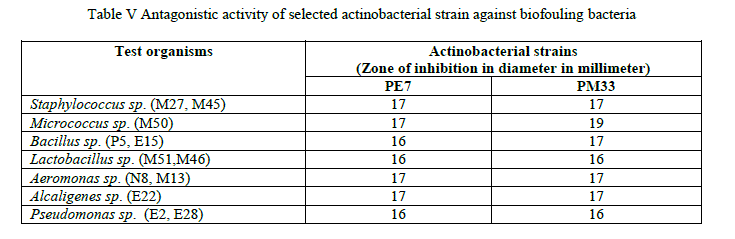 |
| F. Production of antifouling metabolite from selected isolates |
| Both the 2 actinomycete isolates showed good growth on both YEME agar and YEME broth. YEME agar plugs of potential strains showed good activity against Staphylococcus sp. and Alcaligenes sp., respectively (Table 6). |
 |
| The cell free supernatants of 2 actinomycetes showed moderate activity against Staphylococcus sp. and Alcaligenes sp. When compared with liquid fermentation, solid state fermentation showed good growth and promising activity till end of 12th day. |
IV. DISCUSSION |
| In the present study, wide range of bacterial genera was isolated from the biofouling samples. In previous studies, Bacillus sp., Pseudomonas sp. and Staphylococcus sp. (Sarala et al. 2011), Vibrio sp. (You et al., 2007), Aeromonas sp., Micrococcus sp. (Murugan and Santhana Ramasamy, 2002) and Alcaligens sp. (Mary et al., 1993) were frequently isolated from marine biofouling samples and they clearly reported that all the above bacteria able to form biofilm. But there is no report on Kurthia species from biofouling samples. There are different methods used to study the biofilm formation by environmental (Kokare et al., 2009) as well as clinical bacterial pathogens (Mathur et al., 2006). Evaluation was done to confirm the biofilm forming capacity of all the bacteria were isolated from biofouling samples. Out of 55 bacteria 25 bacteria were showed positive for biofilm formation. Isolates which are failed to produce positive results in plate method were also showed strong biofilm formation in tube method. Hence, tube method is more advisable to study the biofilm formation in terms of cost and sensitivity when compared to plate method. There are many reports on actinomycetes from Parangipettai and Pitchavaram mangrove ecosystems with special reference to their antagonistic activity (Balagurunathan et al., 2001; Sivakumar et al., 2007; Kathiresan et al., 2005; Dhanasekaran et al., 2009) and enzymatic potential (Radhakrishnan et al., 2010). But there are very few reports on antifouling activity of actinomycetes from Parangipettai and Pitchavaram marine ecosystems (Kumaran et al., 2010; Bavya et al., 2011). Of the 50 actinomycetes screened, 84 % of the isolates inhibited one or more number of biofouling bacteria. In that PE 7 and PM 33 actinobacterial strains were showed maximum zone of inhibition against more number of gram positive and gram negative biofouling bacteria. |
| Antibiotics are the secondary metabolites produced mainly by filamentous bacteria at the end of stationary phase (Joshi et al., 2012). Organisms which are started to produce secondary metabolites may vary with strain to strain (Antoun, 2005). In case of marine actinobacteria, effect of sea water on secondary metabolite production was well documented (Mitra et al., 2008). But the effect of medium consistency was not yet reported. In the present study, both the potential actinobacterial strains produced the bioactive metabolites very earlier in the YEME agar with 50 % sea water. Most of the antibiotics reported from marine actinobacteria were produced by submerged fermentation (Sambamurthy and Ellaiah, 1974; Balagurunathan and Subramanian, 1993; Wang et al., 2010). But in the present study, solid medium as well as sea water was found to influence the bioactive metabolite production by the actinobacterial strains. This observation also confirmed the marine origin of the actinobacterial strains. Although many natural marine compounds with antifouling activity were isolated from bacteria (Ortega- Morales et al., 2008), algae (Iyyaparaj et al., 2012), fungi (Kwong et al., 2006) and sponges (Henrikson et al., 1995), there are very few compounds like diketopiperazines (Li et al., 2006; Cho et al., 2012) with antifouling activity was reported from marine actinobacteria. In particular, there is no antifouling compound was isolated from marine actinobacterial from India, though few authors reported antifouling activity of marine actinomycetes (Kumaran et al., 2010; Bavya et al., 2011). With this view, two actinobacterial strains PE7 and PM33 reported in this study is a newly added source for the isolation of novel antifouling compounds. Further isolation and characterization of active compound from both the strains and its evaluation against macrofouling organisms like molluscs will lead to the development of novel antifouling candidates. |
V. CONCLUSION |
| The present study is successful in identifying the marine actinomycetes from southeast coast of India which is unexploited for antifouling compounds. This study narrated the characterization and biofilm activity of different biofouling bacteria isolated from different coastal areas of Tamil Nadu, India. Therefore, the potential strains PE7 and PM33 will be a promising antifouling agents and both are combine together with knowledge of coating technology can be utilized for developing eco-friendly antifouling alternatives in future. |
ACKNOWLEDGMENT |
| The authors are thankful to Vice Chancellor and Registrar of Periyar University for providing the facilities and our sincere thanks to UGC (F.No. 37-303/2009 (SR)) for financial assistance by the way of research project. |
References |
|