ISSN: 2319-9865
ISSN: 2319-9865
Department of TB and Respiratory Medicine, Belgaum Institute of medical sciences, Belgaum. Karnataka, India
Received: 15/01/2013 Accepted: 14/02/2013
Visit for more related articles at Research & Reviews: Journal of Medical and Health Sciences
Classical Dengue or ―break-bone fever‖ has been known in India for a very long time. It is an acute viral infection, by at least 4 serotypes (DEN1, 2, 3 and 4) of dengue virus. Of all the arthropod-borne viral diseases, dengue fever is the most common. Dengue fever is one of the most important emerging diseases of the tropical and subtropical regions, affecting urban and periurban areas. Dengue fever can occur epidemically or endemically. Epidemics may be explosive and often start during the rainy season when the breeding of the vector mosquitoes (e.g., Aedes aegypti) is generally abundant. The geographical distribution of the disease has greatly expanded and the number of cases has increased dramatically in the past 30years. A pandemic in 1998, in which 1.2 million cases of dengue fever and dengue haemorrhagic fever were reported from 56countries, was unprecedented. Preliminary data for 2001 indicates a situation of comparable magnitude. However, only a small proportion of cases were reported to WHO; it is estimated that each year 50 million infections occur, with 5,00,000 cases of dengue haemorrhagic fever and at least 1200 deaths, manly among children, although fatalities could be twice as high. The increase of dengue and dengue hemorrhagic fever is due to uncontrolled population growth and urbanization without appropriate water management. The global spread of dengue via travel and trade has made every country to be aware about this threat. This article details the dengue fever with its fatal form in order to reduce the morbidity by the help of various prevention programmes.
Dengue shock syndrome, Dengue haemorrhagic fever, Break bone fever.
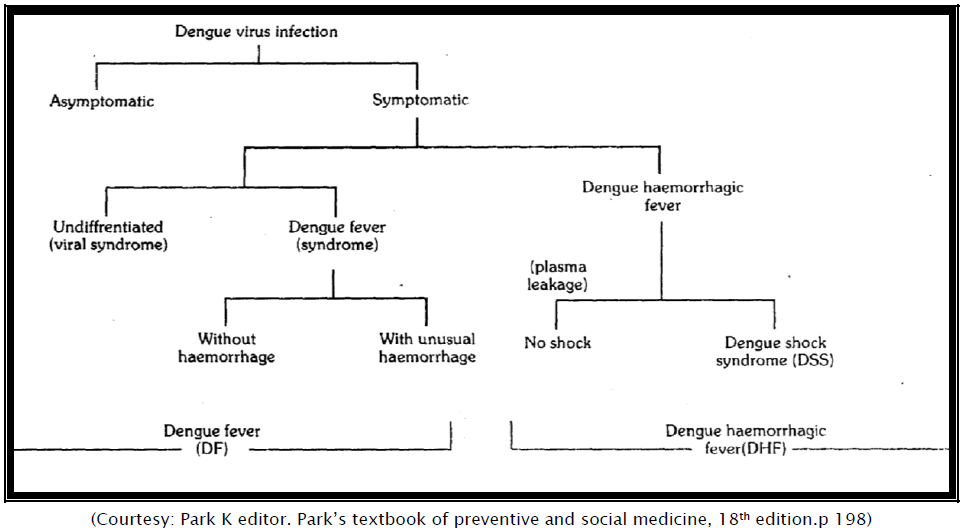
Dengue is a mosquito-transmitted acute viral illness caused by any of the four dengue virus serotypes (DEN-1, DEN-2, DEN-3, and DEN-4). Dengue is endemic in most tropical and subtropical areas of the world [1]. Dengue viruses are arboviruses capable of infecting humans, and causing disease. These infections may be asymptomatic or may lead to a) ―classical‖ dengue fever, or (b) dengue haemorrhagic fever without shock, or (c) dengue haemorrhagic fever with shock. Dengue fever is a self-limiting disease and represents, the majority of cases of dengue infection. A prevalence of Aedes aegypti and Aedes albopictus together with the circulation of dengue virus of more than one type in any particular area tends to be associated with outbreaks of DHF/DSS [2]. The aim of the present article is to enlighten in detail about different forms of dengue diseases, their prevention modalities and their management in order to reduce the morbidity rate by awakening health professionals.
An estimated 100 million cases of dengue fever and 2, 50, 000 cases of dengue haemorrhagic fever occurs annually worldwide [3]. The past 20 years have seen a dramatic geographic expansion of epidemic dengue fever from Southeast Asia to the South Pacific Islands, the Caribbean, and the Americans. An increasing number of reports of dengue fever and associated illness among travellers to dengue virus infected areas paralleled the changing epidemiology of dengue in local populations.
Currently, DF/DHF is endemic in Bangladesh, India, Indonesia, Maldives, Myanmar, Srilanka and Thailand and approximately 1.3 billion people are living in the endemic areas. During the last 15 years, all four dengue subtypes have been isolated in India, Indonesia, Myanmar and Sri Lanka and DEN-2 and DEN-3 have been reported in Maldives and Bangladesh [4].
Approximately 8 percent of travelers to the developing world require medical care during or after travel. Current understanding of morbidity profiles among ill returned travelers is based on limited data from the 1980s [5].
Dengue virus infection can be asymptomatic or cause illnesses ranging from mild undifferentiated fever to severe disease, including hemorrhagic manifestations and shock [6]. Dengue hemorrhagic fever (DHF) is characterized fever, minor or major bleeding phenomena, thrombocytopenia (<100,000 platelets/mm3), and evidence of increased vascular permeability (e.g., hemoconcentration [hematocrit increased by >20% from baseline], pleural or abdominal effusions, or hypoproteinemia). Dengue shock syndrome (DSS) is DHF with signs of circulatory failure, including narrow pulse pressure (<20 mmHg), hypotension, or shock, and might result in a case fatality rate of approximately 10% [7].
Health problems are self-reported by 22 to 64 percent of travelers to the developing world [8]; most of these problems are mild, self-limited illnesses such as diarrhea, respiratory infections, and skin disorders. More importantly, each year, up to 8 percent of the more than 50 million travelers to these regions, or 4 million persons, are ill enough to seek health care either while abroad or on returning home [8-10].
Much of the current understanding of morbidity profiles among ill returned travelers is based on data that were collected in the 1980s [8-11]. Although some morbidity studies have been designed to examine individual diseases [12-16], specific high-risk destinations [17] and certain types of travelers [18-20] a comprehensive, multicenter comparison of the spectrum of illnesses acquired by a broad range of travelers returning from developing regions on all continents has been lacking [21].
Geo Sentinel sites are specialized travel or tropical-medicine clinics on six continents that collect clinician-based surveillance data on travel-related diseases. These data include a broad sample of travel destinations and of morbidity among persons who have become ill while traveling. The 30 current Geo Sentinel sites are specialized travel or tropical-medicine clinics on six continents. They are recruited, on the basis of demonstrated training and experience and a record of publication in travel or tropical medicine, to contribute clinician-based sentinel surveillance data on all ill returned travelers [22].
Over the past 10-15 years, next to diarrhoeal disease and acute respiratory infections, dengue has become a leading hospitalization and deaths, among children in the South East Asia Region. In addition, DHF is increasing and spreading to new areas. The estimated number of annual dengue cases range between 20-30 million and DHF cases about 2, 00, 000 [4]. However, the number of DHF cases reported by endemic countries is substantially lower. The case fatality rates are high in major endemic countries.
The countries in the SEAR have been divided into four Categories. Category A (Indonesia, Myanmar and Thailand): major public health problem, leading cause of hospitalization and deaths among children, spreading to rural environments, multiple virus serotype circulating Aedes aegypti, principal epidemic vector and role of Aedes albopictus uncertain. Category B (India, Bangladesh, Maldives and Sri Lanka): DHF an emerging disease, cyclical epidemics becoming more frequent, multiple virus serotype circulating, expanding geographically within the country, Aedes aegypti principal epidemic vector and role of A albopictus uncertain. Category C (Bhutan and Nepal) no reported cases and endemicity uncertain. Category D (DPR Korea): non-endemic [4].
Classical Dengue or ―break-bone fever‖ has been known in India for a very long time. Temperature also plays an important role in the transmission of dengue virus by mosquitoes. Mosquitoes kept at 26°C fail to transmit DEN-2 virus [2]. Hence, the low incidence of DHF in certain seas6ns could be explained by this observation. The reservoir of infection in both man and mosquito. The Transmission cycle is Man-Mosquito-Man. Aedes aegypti is the main vector. Dengue outbreak has also been attributed to Aedes albopictus, Aedes polynesiensis, and several species of the Aedes scutellaris complex. The Aedes mosquitos become infective by feeding on a patient from the day before onset to the 5th day (viraemia stage) of illness. After an extrinsic incubation period of 8 to 10 days, the mosquito becomes infective, and is able to transmit the infection. Once the mosquito becomes infective, it remains so for life. Transovarian transmission of dengue virus has been demonstrated in the laboratory. All ages and both sexes are susceptible to dengue fever. Children usually have a milder disease than adults. The illness is characterized by an incubation period of 3 to 10 days (commonly 5-6 days). The onset is sudden with chills and high fever, intense headache, muscle and joint pains, which prevent all movement within 24 hours retro-orbital pain particularly on eye movements or eye pressure and photophobia develops. Other common symptoms include extreme weakness, anorexia, constipation, altered taste sensation, colicky pain and abdominal tenderness, dragging pain in inguinal region, sore throat and general depression. Fever is usually between 390C and 40°C. Fever is typically but not inevitably followed by a remission of a few hours to 2 days (biphasic curve). The skin eruptions appear in 80 per cent of cases during the remission or during second febrile phase which lasts for 1-2 days. The rash is accompanied by similar but milder symptoms. The rash may be diffuse flushing, mottling or fleeting pin-point eruptions on the face neck and chest during the first half of the febrile period and a conspicuous rash, which may be maculopapular or scarlatiniform on 3rd or 4th day. It starts on the chest and trunk and may spread to the extremities and rarely to the face. It may be accompanied by itching and hyperesthesia. The rash lasts for 2 hours to several days and may be followed by desquamation [2]. Fever lasts for about 5 days, rarely more than 7 days after which recovery is usually complete although convalescence may be protracted [23]. The case fatality is exceedingly low. Infection with one dengue serotype gives immunity against that particular serotype and partial protection against others.
Dengue haemorrhagic fever (DHF) is a severe form of dengue fever, caused by infection with more than one dengue virus. The severe illness is thought to be due to double infection with dengue viruses. The first infection probably sensitizes the patient, while the second appears to produce an immunological catastrophe [24].
DHF is transmitted by A aegypti. Following an incubation period of four to six days, the illness with high fever accompanied by facial flushing and headache. Anorexia, vomiting, epigastric discomfort, tenderness. The right costal margin and generalized abdominal pain are common. During the first few days the illness usually resembles classical DF, but maculopapular rash usually rubelliform type, is less common. It may appear early or late in the course of the illness,. Occasionally, the temperature may be 40°C to 41°C and febrile convulsions may occur particularly in infants [2]. The major pathophysiologic changes that determine the severity of disease in DHF and differentiate it from DF are plasma leakage and abnormal haemostasis, as manifested by rising haematocrit value and moderate to marked thrombocytopenia. These two clinical laboratory changes are distinctive and constant findings.
Dengue fever is typically a self-limited, if incapacitating, illness, but a life-threatening hemorrhagic form (dengue hemorrhagic fever [DHF]) may complicate infection, especially in children. The incidence of DHF has been linked to the introduction of new DENV serotypes into a region where dengue is endemic, with the sequence of DENV-1 infection followed by DENV-2 infection being particularly associated with more virulent Southeast Asian dengue epidemics [25]. Asian genotype DENV-2 strains have been displacing the relatively more benign American genotype DENV-2 strains in the Americas, notably, accounting for the catastrophic 1981 DENV-2 epidemic in Cuba that followed an uneventful DENV-1 outbreak on the island 4 years earlier [26].
With the rising popularity of international travel to exotic locations, family physicians are encountering more febrile patients who recently have visited tropical countries. In the majority of cases, the fever is caused by a common illness such as tracheobronchitis, pneumonia, or urinary tract infection. However, fever in returned travelers always should raise suspicion for a severe or potentially life-threatening tropical infection. In addition to the usual medical history, physicians should obtain a careful travel history, a description of accommodations, information about pretravel immunizations or chemoprophylaxis during travel, a sexual history, and a list of exposures and risk factors. The extent and type of lymphadenopathy are important diagnostic clues. Altered mental status with fever is an alarm symptom and requires urgent evaluation and treatment. Malaria must be considered in patients who traveled even briefly within an endemic area. Enteric fever is treated with fluoroquinolones, dengue fever with supportive measures only, leptospirosis with penicillin or doxycycline, and rickettsial infections with doxycycline. Estimates indicate that 15 to 37 percent of short-term travelers experience a health problem during an international trip, and up to 11 percent of returned travelers have a febrile illness [27,28].
The majority of travelers with fever have infections that are common in nontravelers, such as upper respiratory tract infection, urinary tract infection, or community-acquired pneumonia. Once routine infections have been considered, the differential diagnosis should be expanded to include travel-related infections. The most serious cause of fever in travelers returning from the tropics is Plasmodium falciparum malaria, which can be rapidly fatal [29,30]. Other important causes of fever in returned travelers include typhoidal and non-typhoidal salmonellosis, dengue fever, viral hepatitis, and rickettsial infections.
The following clinical manifestations have been selected as indicating aclinical diagnosis of DHF without shock [31].
Clinical diagnosis where patients give history of fever with acute onset, high, continuous, and lasting 2-7 days, haemorrhagic manifestations, including at least a method using positive tourniquet test. In DHS, the test usually gives positive result, i.e., more than 20 square. The test usually becomes positive, if the test is done after recovery from shock.
Patient often manifest with petechiae, purpura, ecchymosis, epistaxis, gum bleeding, haematemesis and/or melaena, enlargement of liver Grading of severity of DHF.
The severity of DHF has been classified into four grades according to two pathological hallmarks - shock an bleeding.
Grade I: Fever accompanied by non-specific constitutional symptoms. The only haemorrhagic manifestation is a positive tourniquet test.
Grade II: Patient with spontaneous bleeding usually in the form of skin and/or other haemorrhages in addition to the manifestations in grade I.
Grade III: Circulatory failure manifested by rapid and weak pulse, narrowing of pulse pressure (20 mm Hg or less) or hypotension with the presence of cold clammy skin and restlessness.
Grade IV: Profound shock with undetectable blood pressure and pulse.
The presence of thrombocytopenia with concurrent haemoconcentration differentiates grade I and grade II DHF from DF and other diseases.
Laboratory diagnosis
(a) Thrombocytopenia (100,000/mm3 or less)
(b) Haemoconcentration; haematocrit increased by 20 per cent or more of baseline value.
The first two clinical criteria plus thrombocytopenia and haemoconcentration or a rising haematocrit are sufficient to establish a clinical diagnosis of DHF [31].
The clinical diagnosis is based on:
(a) All the above criteria, plus
(b) Shock-manifested by rapid and weak pulse with narrowing of the pulse pressure (20 mm Hg or less) or hypotension, with the presence of cold, clammy skin and restlessness.
The management [2] of dengue fever is symptomatic and supportive. Bed rest is advisable during acute febrile phase. Antipyretics or sponging are required to keep the body temperature below 40 C. Aspirin should be avoided particularity in areas where DHF is endemic, since it may cause gastritis, bleeding and acidosis. Oral fluid and electrolyte therapy is recommended for patients with excessive sweating, vomiting diarrhoea.
The management of DHF during the febrile phase is similar to that of DF. A rise in haematocrit value indicates significant plasma loss and a need for parenteral fluid therapy. In grade I and II, volume replacement can be given for a period of 12-24 hours. Patients with any signs of bleeding and persistently high haematocrit values, despite being given volume replacement should be admitted to hospital. The volume and type of fluid should be similar to that used in the treatment of diarrhoea with moderate isotonic dehydration, but the rate should be carefully titrated. The required fluid volume should be charted on a two to three hourly basis and the rate of administration adjusted throughout the 24 - 48 hour period of leakage. Serial haematocrit determination, every four to six hours, and frequent recording of vital signs are recommended for adjusting the fluid replacement in order to assure adequate volume replacement and avoid over-transfusion.
The fluid replacement should be the minimum volume that is sufficient to maintain effective circulation during the period of leakage. Excessive replacement will cause respiratory distress (from massive pleural effusion and ascites), pulmonary congestion and oedema.
The types of fluids used are
Crystelloid: Five per cent dextrose in lactated Ringer’s solution [32], five per cent dextrose in acetated Ringer’s solution five per cent dextrose in half strength normal saline solution and five per cent dextrose in normal saline solution.
Colloidal: Dextran 40 and Plasma
Management of Shock
DSS is a medical emergency that requires prompt and vigorous volume replacement therapy. There are also electrolyte (sodium) and acid-base disturbances. It must be considered that there is a high potential for developing disseminated intravascular clotting (DIC) and that stagnant acidaemia blood will promote and or enhance DIC, which may lead to severe haemorrhage and/or irreversible shock. Replacement of plasma loss: Immediate replacement of plasma loss with isotonic salt solution (five per cent dextrose in acetated Ringer’s solution or five per cent dextrose in normal saline solution) at the rate of 10-20 ml/kg body weight/h, or, in the case of profound shock (grade IV), as a bolus of ten mi/kg body weight (one to two times) should take place. In case of continued or profound shock (with high haematocrit values), colloidal fluid (dextran or medium molecular weight in NSS or plasma) should be given fottowing the initial fluid at a rate of 10—20 ml/kg body weight/h. Blood transfusion is indicated in cases with profound or persistent shock despite declining haematocrit values after initial fluid replacement. When improvement is apparent, the rate of IV fluid replacement should be reduced and adjusted one or two hourly, throughout the 24-hours period. Colloidal fluid is indicated in cases with massive leakage, and to whom a large volume of crystalloid fluid has been given.
In small children, five per cent dextrose in a half-strength normal saline solution (5 per cent D/1/2 NSS) is used following initial resuscitation, and 5 pr cent D/1/3 NSS may be used in infants under one year of age, if the serum sodium is normal.
Intravenous fluid should be discontinued when the haematocrit reading [32] drops to around 40 per cent and vital signs are stable. A good urine flow indicates sufficient circulating renal volume. A return of appetite and diuresis are signs of recovery. In general, there is no need for fluid therapy for more than 48 hours after onset of leakage and/or shock reabsorption of extravasated plasma takes place one or two days thereafter (manifested by further drop in haematocrit after IV fluid has been stopped and clearing of pleural effusion and ascites has occurred) and may cause hypervolaemia,heart failure and pulmonary oedema if more fluid is given. It is extremely important to emphasize that a drop in haematocrit reading at this stage should not be interpreted as a sign o internal hemorrhage. Strong pulse and blood pressure with wide pulse pressure and diuesis are good vital signs during this reabsorption phase.
Dengue/DHF is widely prevalent in India, and all the 4 serotypes are found in the country. It is reported from 15 states since 1996. During 2003 there were 12750 cases with 217 deaths from dengue in the country. Since 1996, National Antimalarial Prevention programme has been monitoring dengue situation in the country. Government of India has issued certain guidelines on prevention and control of dengue [32].
Persons traveling to areas where dengue is endemic should avoid exposure to mosquitoes by using repellents, wearing protective clothing, and remaining in well-screened or air-conditioned areas. No vaccine is available for preventing dengue infection. Health-care providers should consider dengue in the differential diagnosis of illness for all patients who have fever and a history of travel to tropical areas within 2 weeks before the onset of symptoms. Supportive measures should be administered, and only acetaminophen is recommended for management of pain and fever. Acetylsalicylic acid (i.e., aspirin) and other nonsteroidal anti-inflammatory agents are contraindicated because of their anticoagulant properties. Dengue patients should be monitored for signs of DHF, especially hypotension, because prompt fluid therapy reduces morbidity and mortality [1].
Mosquito control
The vector of DF and DHF (eg, A. aeg,jptj) breed in and around houses and in principle can be controlled by individual and controlling antilarval measures.
Vaccines
So far, there is no satisfactory vaccine and no immediate pjospect of preventing the disease by immunization.
Other measures
Isolation under bed nets during the first few days; individual protection against mosquitoes.