ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Sakshi Kabra1, Stuti Katara 2, Ashu Rani3*
|
| Corresponding Author: Ashu Rani, |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
An examination of the structural, morphological and mineralogical features of Turkish perlite sample was carried out by FT-IR, SEM, SEM-EDX, XRD, TGA and N2-adsorption-desorption analytical techniques. Perlite is a natural, silica enriched igneous rock. This article features the primary step of study of basic characterization of perlite to investigate its utilization in different areas. Higher silica and alumina content was shown by EDX analysis. Both XRD and FT-IR studies reveal the presence of amorphous silica in perlite. TGA and LOI analysis illustrate the presence of water in the sample. The irregular morphology was exposed through SEM images. It was evident from the results that silica-enriched perlite could be developed further into a potential material in several area including catalyst synthesis by using various activation techniques.
Keywords |
| perlite, characterization, XRD, FT-IR. |
INTRODUCTION |
| The basic objective of this paper is to provide the comprehensive characterization of Turkish perlite and suggest its suitability towards several fields of utilization. Perlite is a hydrated, widely occurring amorphous igneous rock formed by cooling of volcanic eruptions. Its unique structure consists of numerous concentric layers having SiO2, Al2O3, K2O and Na2O as major constituents while TiO2, CaO, MgO, Fe2O3 and hydrated water as well as unburned carbon remain present in varying quantities [1]. On heating the perlite to its softening range, i.e., above 850°C, crystalline water molecules vaporize and escape resulting in unusual expansion of perlite up to 7-16 times of its original volume, creating inert, non-toxic, lightweight particles with specific surface area of about 1.22 m2g-1 [2], density in the range of 0.6 - 2.30 gml-1 [3] and particle size in range of 0.2-4 mm. [4]. Physio-chemical properties of perlite need to be examined prior to evaluation of its possible utilization in various fields. As far as applications of perlite are concerned, it is mainly consumed as fillers, filter aids, in producing building construction materials [5], [6], adsorptive materials, precursor for geo-polymer formation [7], removal of heavy metal ions and other pollutants from atmosphere [8], in thermal insulation [9], removal of dyes [10], [11], in horticulture [12], sorption of oil [4] etc. Perlite is a naturally occurring waste, estimating about 700 million tons worldwide reserves. Turkey has rich resources of perlite, approximately 160 tons [13], since, domestic demand is very limited, so, most of the produced perlite is exported to various countries including India. Thus its effective, conducive and eco-friendly utilization has always been a challenge for scientific community. Above applications could thrive up to only some level in utilizing the huge reserves of perlite. However, the search of new applications of the perlite as either catalyst or catalyst support material is still enduring. Literature accounts only for the few applications of perlite as photo catalyst [14], pyrolysis catalyst [15] and catalyst used for different chemical reactions such as, immobilization [16], methacrolein production [17], zeolite synthesis [18], [19] etc. The objective of the present work is to characterize perlite, in terms of structure, mineralogical composition, chemical behaviour, colour and morphology. This is the foundation stone in our research work to investigate the physio-chemical properties of perlite for its utilization in different fields. This paper mainly deals with identification of characteristics of perlite and their study using spectroscopic and microscopic analysis through FT-IR, SEM, SEMEDX, XRD, TGA and N2-adsorption-desorption techniques. |
EXPERIMENTAL |
| A. Materials |
| Perlite sample was imported from Turkey and supplied to us by Indica Chem. Ind. Pvt. Ltd., India. |
| B. Procedure |
| `Perlite sample was taken as such and thermally treated in a muffle furnace under static conditions at 800°C for 3 h and named as TAP. |
| C. Characterization |
| Physiochemical properties of perlite and TAP were studied by FT-IR, SEM and SEM-EDX, XRD, TGA, and N2 adsorption-desorption studies. The FT-IR study of the sample was done by Bruker FT-IR Spectrophotometer (TENSOR 27) in DRS mode by mixing samples with KBr in 1:20 weight ratio. The spectra were recorded in the range 550-4000 cm-1 with a resolution of 4 cm-1. SEM (Model-JEOL-JSM 5600) was used to investigate morphology and surface texture of the sample while elemental analysis was studied by SEM-EDX analysis (Model-INCA Oxford). X-ray diffraction study was done by Xray powder diffractometer (Bruker D8 Advance) using Cu Kα radiation (λ = 1.5406AÃâ¹ÃÅ¡). The samples were scanned in 2θ range of 5-65Ãâ¹ÃÅ¡ at a scanning rate of 0.04Ãâ¹ÃÅ¡s-1. TGA of the sample was carried out using Mettler Toledo thermal analyser (TGA/DSC1 SF/752), by heating the sample in the range of 50–850 °C with a heating rate of 10 °C/min under nitrogen flow (50 cm3/min). BET surface area of the samples was measured at liquid nitrogen temperature (77 K) using Quantachrome NOVA 1000e surface area analyser. The samples were degassed under vacuum at 120 °C for 2 h, prior to measurement. |
RESULTS AND DISCUSSION |
| Perlite is white or light-gray coloured material which turns to light pinkish on thermal treatment. FT-IR spectrum of perlite and TAP (Fig. 1) shows a broad band between 3600-3300 cm-1, which is attributed to surface –OH groups of – Si-OH and water molecules adsorbed on the surface. The broadness of band indicates the presence of strong hydrogen bonding in the sample [20]. The hydroxyl groups exist in higher degree of association with each other which results in extensive hydrogen bonding, while in FT-IR spectrum of TAP, the intensity and broadness of band is decreased which confirms the loss of water (Fig. 1b). The strong band at 1178 cm-1 is due to the structural siloxane framework, which is the vibrational frequency of the Si-O-Si bond. The peak is shifted to higher wave number, i.e., 1192 cm-1 after thermal treatment, which is normally observed in amorphous silica samples [21]. An intense peak at 1633 cm-1 in the spectrum of perlite is attributed to bending mode (δO-H) of water molecule [22], which is again highly decreased in case of TAP. The region around 805 cm-1 is characteristic of Si-O-Si symmetric stretching modes [23], [24]. Amorphous silica exhibited a relatively strong peak at about 800 cm–1 and it can be distinguished from the band of crystalline silicate [25]. The observed frequencies of IR bands of perlite and their possible assignments are summarized in Table 1. |
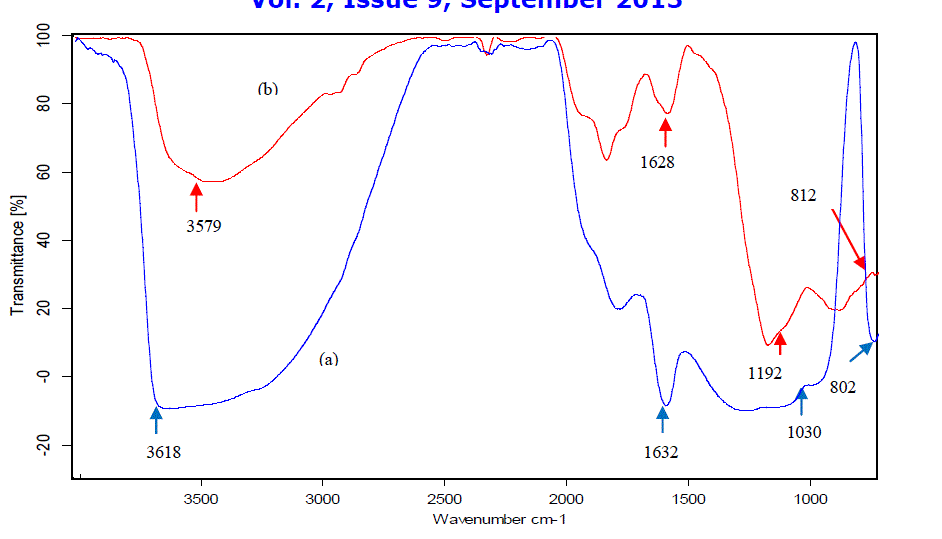 |
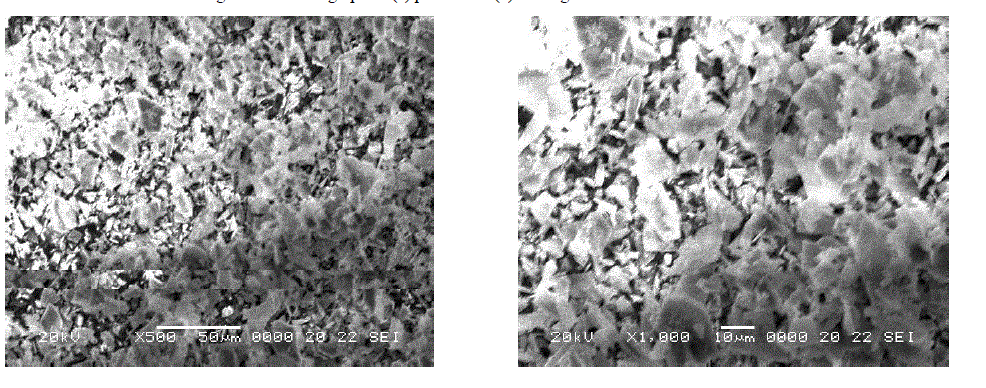
| SEM micrograph of perlite and its magnified view (Fig. 2 a and b) revealed the irregular morphology of perlite particles with broken or ragged edges. Similar pattern was observed in other reported micrographs of perlite [7]. SEM images of TAP and its magnified view (Fig. 3 a and b) are mainly fragmatic and random as a result of thermal activation [4]. But here, the morphology is less irregular which confirms the evaporation of water from the perlite sample on heating at high temperature. |
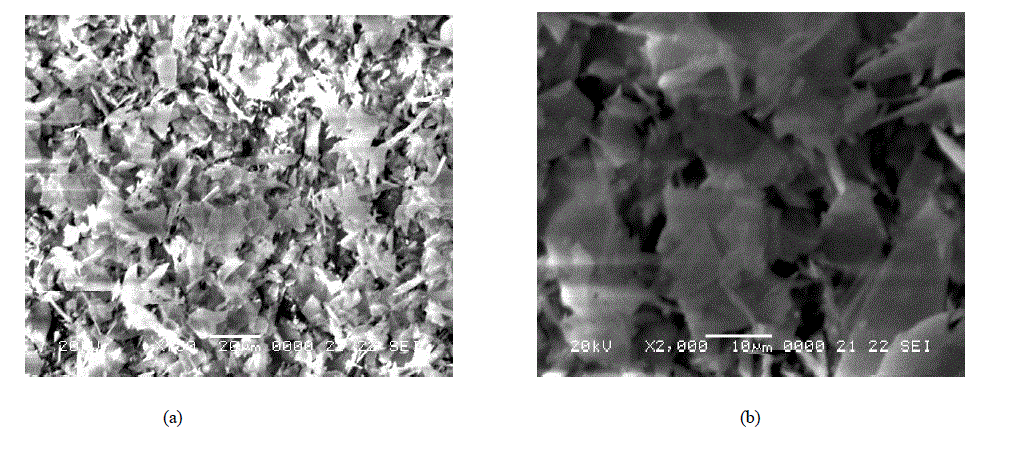 |
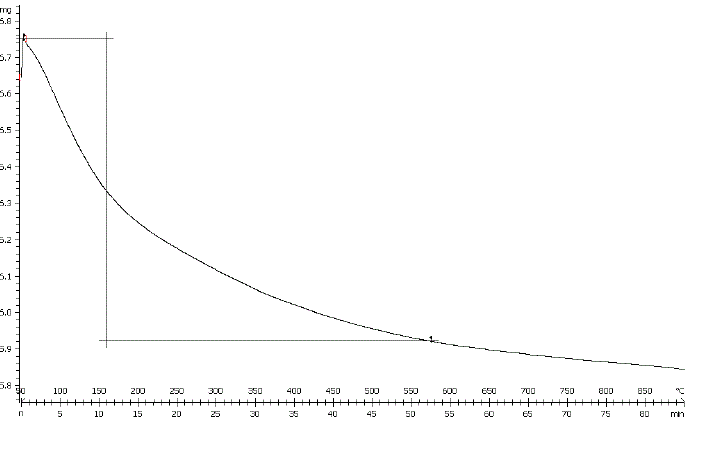
| Loss on ignition (LOI) was determined by heating a certain weighed quantity of perlite in muffle furnace at 800Ãâ¹ÃÅ¡C for 3 h. The LOI amount was 4.1 wt % which corresponds to the removal of moisture and coexisting unburned carbon from sample [26]. The TGA curve of perlite, as shown in Fig. 4, shows continuous decrease in weight of sample from 50- |
| 900°C. Weight loss in lower temperature range relates to the removal of moisture content of the sample together with some volatile materials. While, the weight loss of sample within range of 550-900Ãâ¹ÃÅ¡C would correspond to the burning of carbonaceous materials that were firmly adsorbed on the surface of the solid materials remaining or volatilization of some trace metal oxides [27]. |
 |
| BET surface area of perlite and TAP samples were found to be 2.6 and 2.3 m2/g respectively. The broad powder X-ray diffraction pattern of perlite (Fig. 5a), confirmed the absence of any ordered crystalline structure [28] which is typical for amorphous solids. However, heating of perlite at temperature over 800Ãâ¹ÃÅ¡C for 3 h could convert less ordered structure to a more highly ordered structure and a single crystalline peak appears at 2θ = 27.642Ãâ¹ÃÅ¡ (Fig. 5b) which shows presence of quartz in the sample [29], along with a broad peak at 2θ = 22-23Ãâ¹ÃÅ¡ confirming amorphous nature of silica [24], [30]. |
 |
CONCLUSIONS |
| Results obtained in the present investigation confirm silica and alumina as major constituents of perlite. It is also observed that the nature of silica in perlite is mainly amorphous with irregular morphology and only a single crystalline peak appears on thermal treatment. On the basis of analysis, it can also be said that high number of hydroxyl groups and Si-O-Si network is also present in perlite. Moreover, comparison of perlite with TAP shows the effects of thermal treatment on structure, mineralogy, colour, surface area and morphology of perlite. The contemporary report aims at study of fundamental characteristics of perlite for its further applications in novel fields. The results like presence of amorphous silica network and surface hydroxyl groups in perlite indicate towards the potential capability of perlite as a support material in catalyst synthesis. |
ACKNOWLEDGEMENT |
| The authors are thankful to Dr. Mukul Gupta, Dr. D.M. Phase, Er. V.K. Ahiray from UGC-DAE CSR Lab, Indore, India for XRD and SEM, SEM-EDX respectively. The financial support was provided by Fly Ash Mission, DST, New Delhi, India. The authors are also grateful to UGC, New Delhi, India for their Junior Research Fellowship scheme. |
References |
|