e-ISSN: 2319-9849
e-ISSN: 2319-9849
Department of Pharmacy, Oxford College of Pharmacy, UPSIDC, Ghaziabad, India
Department of Pharmacy, Banasthali Vidyapith, Rajasthan, India.
Received date: 06/04/2015 Accepted date: 23/04/2015 Published date: 27/04/2015
Visit for more related articles at Research & Reviews: Journal of Chemistry
Oxadiazole is an important heterocyclic compound containing one oxygen and two nitrogen atoms in the five membered ring. 1,3,4-oxadiazole is a versatile heterocyclic nucleus having novel molecule which attract the medicinal chemist to search a new therapeutic molecule. The present review summarizes physicochemical properties, various synthetic procedures and various pharmacological activities of 1,3,4-oxadiazole moiety. 1,3,4-oxadiazole moiety is an important pharmacophore which plays a major role in the pharmaceutical chemistry and broad range of important biological activities such as anti-inflammatory, analgesic, ulcerogenic, antimicrobial, antifungal antitubercular, anticonvulsant, anticancer/antitumor, antiviral, and antihypertensive activities etc. as reported in the literature.
Oxadiazole, Anti-inflammatory, Analgesic, Antitubercular, Anticonvulsant, Anticancer/Antitumor and Antiviral activities.
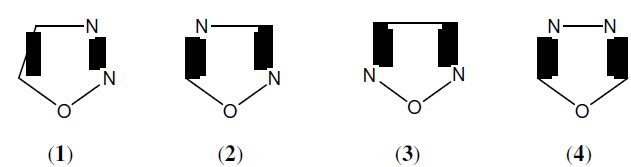
Oxadiazole is important heterocyclic compound containing one oxygen and two nitrogen atoms in five membered ring, which is considered to be derived from furan by the replacement of two methane (‐CH=) group by two pyridine type nitrogen (‐N=). Depending upon the position of N- atom in the heterocyclic ring, oxadiazoles may be divided into four isomers: (1) 1,2,3-oxadiazole, (2) 1,2,4-oxadiazole, (3) 1,2,5-oxadiazole and (4) 1,3,4-oxadiazole [1].
However, 1,3,4-oxadiazole and 1,2,4-oxadiazole are better known, and more widely studied by researchers because of their many important chemical and biological properties. Among heterocyclic compounds, 1,3,4-oxadiazole has become an important construction motif for the development of new drugs. Literature survey revealed that a minor modification in the structure can result in qualitative as well as quantitative changes in the activity, convinced us to begin on the synthesis of various new 1,3,4-oxadiazole derivatives with the aim of having improved activity and lesser toxicity. The synthesis of novel 1,3,4-oxadiazole derivatives and investigation of their chemical properties and biological behaviour has accelerated in the last two decades. In recent years the number of scientific studies with these compounds has increased considerably. Considering the period from 2002 to 2012, the Scifinder Scholar database records 2,577 references to 1,3,4-oxadiazole, demonstrating its relevance for heterocyclic chemistry [2].
1,3,4-oxadiazole derivatives is an important pharmacophore which play a major role in the pharmaceutical chemistry and broad range of important biological activities. We have decided to present the main synthesis approaches used for obtaining the heterocyclic nucleus, as well as the broad spectrum of pharmacological activities such as anti-inflammatory, analgesic, ulcerogenic, antimicrobial, antifungal antitubercular, anticonvulsant, anticancer/antitumor, antiviral, and Antihypertensive activities etc. as reported in the literature.
Physical properties
1,3,4-Oxadiazole (5) is a five member heterocyclic compound having two carbon, two nitrogen, one oxygen and two double bonds.
The first monosubstituted 1,3,4-Oxadiazoles were reported in 1955 by two independent laboratories [3-4]. Since 1955 other workers have extended this reaction 1,3,4-Oxadiazole boils at 150°C [5-7]. The percentage of C,H,N and bond angle present in 1,3,4-Oxadiazole are given in Table 1 & 2 [8]
Chemistry of oxadiazole ring
Oxadiazole is a heterocyclic aromatic chemical compounds having a five member ring containing one oxygen and two nitrogen atoms and molecular formula of oxadiazole C2H2N2O. There are four isomers of oxadiazole: (1) 1,2,3-oxadiazole (2) 2 2 2 1,2,4-oxadiazole, (3) 1,2,5- oxadiazole and (4) 1,3,4-oxadiazole are known, but the 1,2,3-isomer is unbalanced and reverts to the diazoketone tautomer. Name for oxadiazole ring such as ‘Azoxime’ (1,2,4-oxadiazole), ‘Furazan’ (1,2,5-oxadiazole), ̔ Furazans’ (1,2,5-oxadiazole) and ̔ Biazole, oxybiazole’ (1,3,4- oxadiazole) [9].

Oxadiazole is a very weak base due to the inductive effect of the extra heteroatom. The replacement of two –CH=groups in furan by two pyridine type nitrogen (-N=) reduces aromaticity of resulting oxadiazole ring to such an extent that the oxadiazole ring exhibit character of conjugated diene. The electrophillic substitutions in oxadiazole ring are extremely difficult at carbon atom because of the relatively low electron density. It can be attributed to electron withdrawal effect of the pyridine type nitrogen atom. However the attack of electrophiles occurs at nitrogen, if oxadiazole ring is substituted with electron-releasing groups. Oxadiazole ring is generally resistant to nucleophilic attack. Literature survey reveals that the oxadiazoles undergoes number of reactions such as electrophilic substitution, nucleophilic substitution, thermal and photochemical [10].
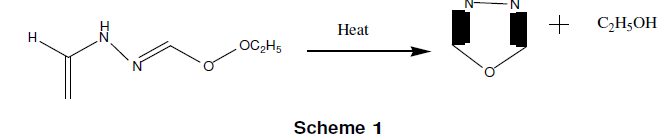
1, 3, 4-oxadiazole is a liquid, which boils at 150°C. Ainsworth first prepared it in 1965 by the thermolysis of ethylformate formly hydrazone at atmospheric pressure as given in scheme-1 [11]
Thermal and photo-chemical reactions (Thermal reaction)
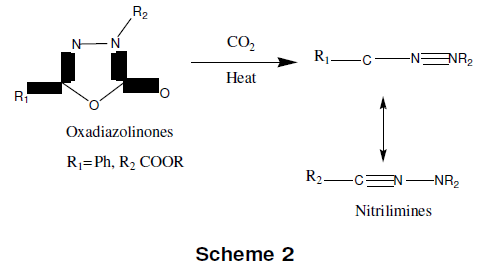
1,3,4-oxadiazole is thermally stable and this stability is increased on substitution, particularly by aryl and perfluro alkyl groups. Oxadiazolinones lose carbon dioxide at high temperature to give nitrelimines. Recyclization in the nitrelimines formed at 210-230°C from oxadiazolinone yields 2-alkoxy-1,3,4-oxadiazole as given in scheme-2 [11].
Loss of Nitrogen
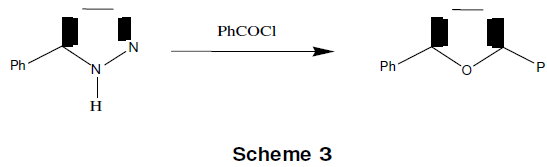
Tetrazoles with acid chlorides (in C5H5 N at 50°C) give 1,3,4-oxadiazole as given in scheme-3 [11].
Reactivity of 1, 3, 4-oxadiazole
As 1,3,4-oxadiazole have a relatively low electron density at carbon (positions 2 and 5) and a relatively high electron density at nitrogen (positions 3 and 4), the major reactions are neucleophillic attack at carbon, generally followed by ring cleavage and electrophilic attack at nitrogen. This reactivity towards nucleophiles, also catalyzed by acid, causes difficulties when carrying out reactions, which involve basic or acidic conditions. This ring is more stable when substituted by one or more aryl groups. Tautomeric oxadiazole react with electrophile at ring nitrogen at the exocyclic heteroatom or at both center. Reactions in the substituent groups of alkyl or aryl 1,3,4-oxadiazole are possible but they are limited by the sensitivity of the ring to the reagent used substituent groups of alkyl or aryl 1,3,4-oxadiazole are possible but they are limited by the sensitivity of the ring to the reagent used [11].
A. Anti-inflammatory and analgesic activity:
Omar FA, et al. synthesized a series of 2,5-disubstituted 1,3,4-oxadiazoles derivatives. Most of the tested compounds (6, 7, 8, 9 and 11) exhibited higher anti-inflammatory activity (6, 9 and 10) exhibited higher analgesic activity and (6, 9 and 11) reduced ulcerogenic activity than ibuprofen, the standard reference drug [12].
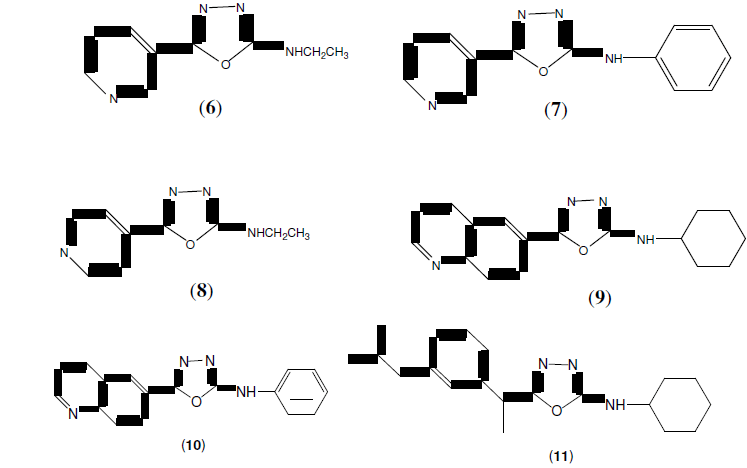
Palaska E, et al. synthesized a series of 2-(2-napthyloxymethyl)-5-Substituted amino-1,3,4-oxadiazole derivatives (12) and evaluated them for their anti-inflammatory activity with reduced side effects [13].
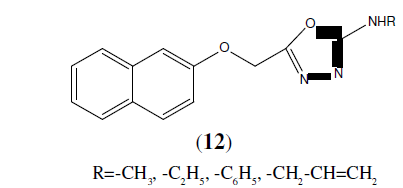
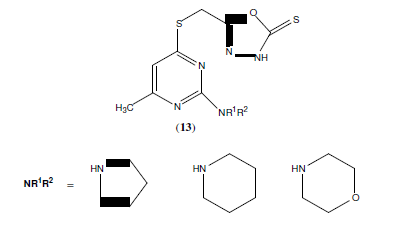
Burbuliene M.M, et al, synthesized a series of 5-[(2-disubstituted amino-6-methyl- pyrimidin-4-yl)-sulfanylmethyl]-3H-1,3,4- oxadiazole-2-thiones (13). All the tested compounds possess anti-inflammatory activity comparable to that of acetylsalicylic acid and some compounds were found to be much more active than ibuprofen [14].
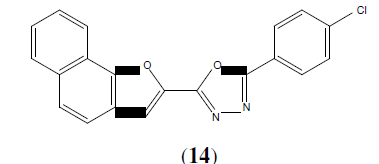
Ravindra KC, et al. synthesized a series of 1,3,4-oxadiazoles linked to naptho[2,1-b] furan derivatives. This compound possesses (14) better anti-inflammatory activity than ibuprofen [15].
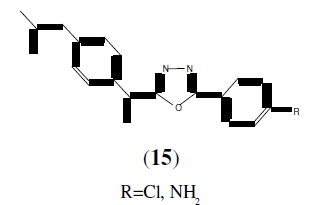
Mohmad A, et al. synthesized a series of newer 2,5-disubstituted-1,3,4-oxadiazole derivatives (15) as potential antiinflammatory activity [16].
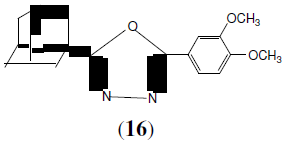
Kadin AA, et al. synthesized a series of 2-(1-adamantyl)-5-substituted-1,3,4-oxadiazole compounds displayed strong dose dependent inhibition of carrageenan-induced paw edema with >50% inhibition at a concentration of 60 mg/kg. The compound (16) with the 3,4-di-MeO group was more potent than the indomethacin standard [17].
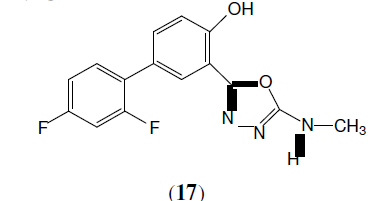
Küçükgüzel SG, et al. synthesized a some novel 1,3,4-oxadiazole compounds (17) derived from diflunisal hydrazide as potential anti-infective and anti-inflammatory agents [18].
Kumar H, et al. synthesized another series of 1,3,4-oxadiazole and 1,2,4-triazole derivatives of biphenyl-4-yloxy acetic acid (18) & screened them for their potent anti- inflammatory activity by using carrageenan induced rat paw edema method. The compounds were found to possess much more anti-inflammatory activity (15.90 to 81.81%) than the reference drug flurbiprofen (79.54%). In addition these compounds also exhibited analgesic activity and low ulcerogenic effect [19].
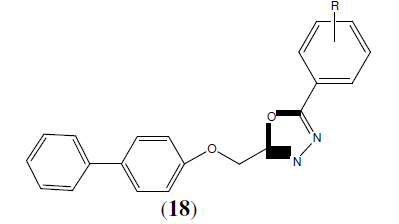
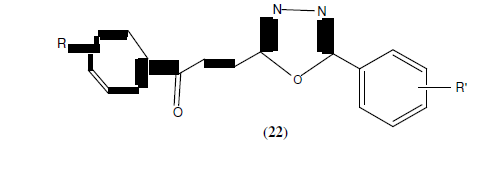
R=4-Cl, 2-Cl, 2,4-dichloro
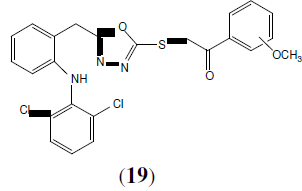
Bhandari SV, et al. synthesized a series of S-substituted phenacryl 1,3,4-oxadiazole and schiffs bases derived from 2-[(2,6-dichloroanilino) phenyl] acetic acid (diclofenac acid). Out of 18 compounds synthesized, eight were found to possess significant anti-inflammatory activity with analgesic activity in acetic acid induced writhing tests with no ulcerogenic activity. The compound (19) has most prominent analgesic activity [20].
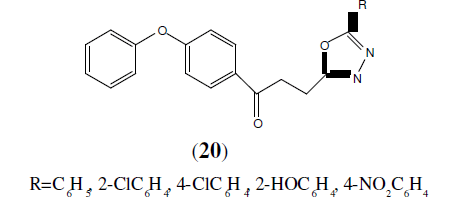
Husain A, et al. 2008 synthesized series of novel 1-(4-phenoxyphenyl)-3-[5-(substituted aryl)-1,3,4-oxadiazol-2-yl]propane-1- ones (20) and screened for analgesic activity. The 2-acetoxy phenyl derivatives of this series have shown 76% analgesic activity which is higher than standard drug indomethacin [21].
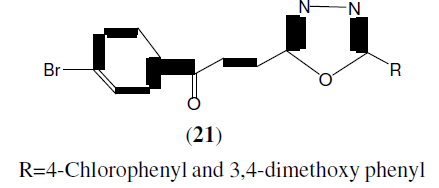
Husain A, et al. synthesized a novel series of 2-[3-(4-bromophenyl)propan-3-one]-5-(substituted phenyl)-1,3,4-oxadiazoles (21) from 3-(4-bromobenzoyl) propionic acid with the aim to get better anti-inflammatory and analgesic drugs with minimum side effects (ulcerogenicity). Two compounds, 2-[3-(4-bromophenyl)-propan-3-one]-5-(4-chlorophenyl)-1,3,4-oxadiazole and 2-[3-(4-bromophenyl)propan-3-one]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole with anti-inflammatory activity of 59.5 and 61.9%, respectively, were found to have comparable activity with that of indomethacin which showed 64.3% activity at the same dose [22].
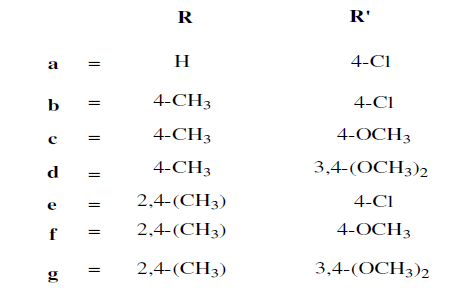
Akhtermymoona et al. reported synthesis of 2,5-disubstituted-1,3,4-oxadiazole derivatives based on Aroyl propionic acid (22). These synthesized compounds were studied for their anti- inflammatory, analgesic, ulcerogenic, and lipid peroxidation. Some of synthesized compounds showed anti-inflammatory activity 81.46% and 89.50% respectively against standard drug ibruprofen [23].
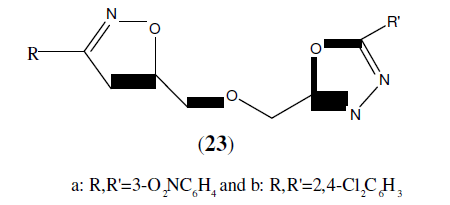
Jayashankar B, et al. synthesized a series of novel ether-linked bis(heterocyclic) (23). All the synthesized compounds were screened for anti-inflammatory and analgesic activities. 23a and 23b showed excellent activity against ibuprofen and aspirin [24].
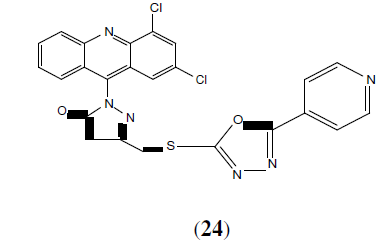
Chandra T, et al. synthesized a series of 1,3,4-oxadiazole derivatives. These compounds were screened for their antiinflammatory and analgesic activity. This compound (24) was found to possess anti-inflammatory activity ranging from 10.8 to 40.8%. In addition this compound also exhibited analgesic activity [25].
Manjunatha AK, et al. synthesized a series of oxadiazole derivatives (25) shows anti-inflammatory activity using paw edema induced by carrageenan as the method with diclofenac sodium as the reference. In addition these compounds also containing 4-Cl, 4-NO2, 4-F and 3-Cl groups were more active than diclofenac sodium, the compound R=4-F showed maximal analgesic activity [26].
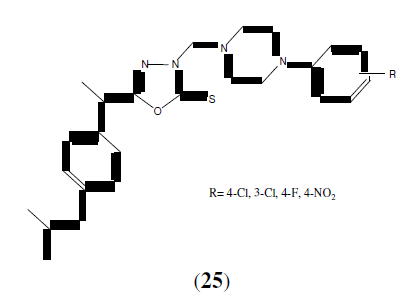
Dewangan D, et al. synthesized a series of some novel 2,5-disubstituted-1,3,4- oxadiazoles (28) shows analgesic activity by using acetic acid induced writhing method as compared to standard drug diclofenac. Potent analgesic activity have been found in bis(heterocyclic) substituted-1,3,4-oxadizole. They also studied SAR of these synthesized compounds. The 2‐position and 5‐ position is an extremely important site of molecular modification, which play a dominant role in determining the pharmacological activities of 1,3,4‐oxadiazole derivatives (26, 27 and 28). The synthesized compounds were screened using carrageenean induced rat paw edema. Direct substitution of the 2‐position with an ‐C5H4N and ‐2‐COOH‐C6H4 , with pyridine in 5‐position enhance the anti‐inflammatory activity of 1,3,4‐oxadiazole derivative [27].
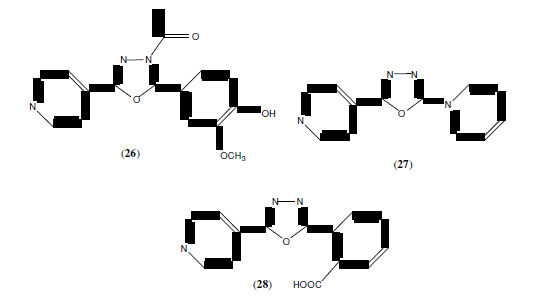
Palusa SKG, et al. synthesized a series of pyrimidine substituted 1,3,4-oxadiazole derivatives (29) and evaluated antiinflammatory activity [28]
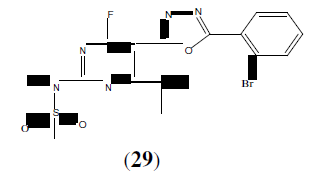
Sivaraj S, et al. synthesized some newer 1,3,4-oxadiazole derivatives of diclofenac (30). Most of these potent ligands were synthesized and screened for analgesic, anti-inflammatory and ulcerogenic potential [29].
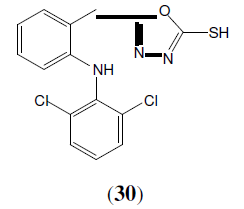
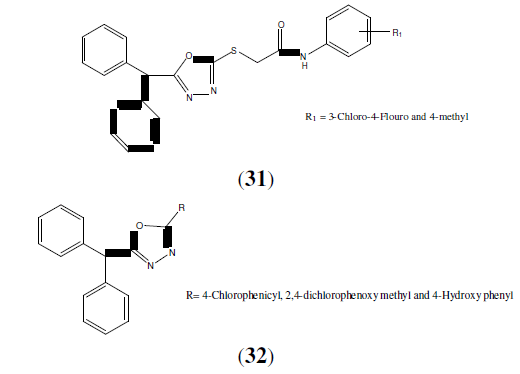
Amir M, et al. synthesized a series of 1,3,4-oxadiazole derivatives of aryl acetic acid. These compounds (31 and 32) evaluated better analgesic and anti-inflammatory activity than standard drug [30].
Nimavat B, et al. synthesized of some novel 1,3,4-oxadiazoles derivatives (33) and evaluated in-vitro anti-inflammatory activity by inhibition of bovine serum albumin denaturation method. The results of the anti-inflammatory activity of these compounds are showed good activity [31].
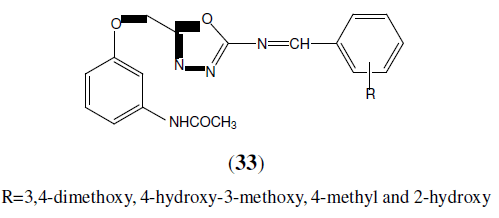
R=3,4-dimethoxy, 4-hydroxy-3-methoxy, 4-methyl and 2-hydroxy
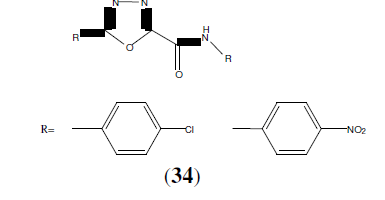
Singh AK, et al. synthesized some 1,3,4-oxadiazole derivatives shows (34) for anti- inflammatory activity. These compounds for maximum anti-inflammatory activity than standard drug [32].
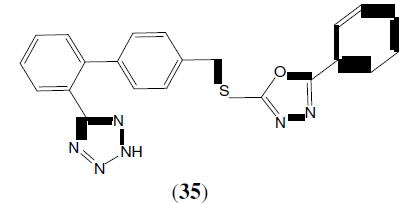
Chao J, et al. synthesized a series of several new 5-[4-(5-phenyl-1,3,4-oxadiazole-2-yl- sulfonylmethyl)-biphenyl-2-yl]-tetrazole derivatives. These compounds screened for their antimicrobial activity against Bacillus subtilis and Escherichia coli at the concentration of 100 μg/mL. This compound (35) showed a better inhibitory of this bacterial growth [33].
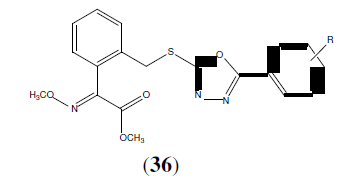
Li Y, et al. Synthesized a series of (E)-V-(methoxyimino)-benzeneacetate derivatives containing 1,3,4-Oxadiazole ring (36) and tested for their fungicidal activities. All the compounds showed potent fungicidal activities against Rhizoctonia solani, Botrytis cinereapers, Gibbereapers zeae, Physalospora piricola and Bipolaris mayclis [34].
A: R=H, B: R=4-OCH3, C: R=4-C6H5-CH O, D: R=3-Cl, E: R=2,3-Cl2, F: R=2,4-Cl2 , G: R=2,5-Cl , H: R=2,4-Cl -5-F, I: R=2-F, 3 6 5 2 J: R=4-F K: R=2-F-4-Br, L: R=2,3-F2
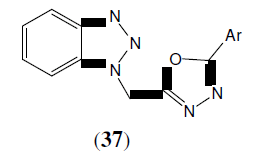
Shahar Yar M, et al. Synthesized a series of some newer 2,5-disubstituted-1,3,4-oxadiazole derivatives. The antimicrobial activity of the synthesized compounds was evaluated, on Staphylococcus aureus and Escherichia coli. Ofloxacin was used as standard in a concentration of 30 μg/disc. This compound (37) showed maximum activity in against Staphylococcus aureus [35].
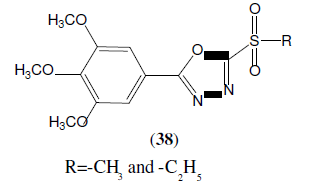
Chen C, et al. synthesized 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-oxadiazole derivatives (38) and tested for their antifungal activity against Gibberella zeae, Botrytis cinerea, Sclerotinia sclerotiorum. These compounds exhibiting promising antifungal activities even better than that of the commercial fungicide drug Hymexazol [36].
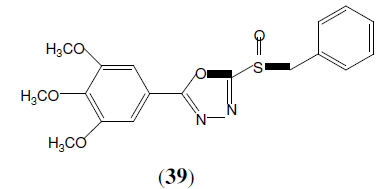
Liu F, et al. synthesized sulfoxide derivatives containing tri-methoxyphenyl substituted 1,3,4-Oxadiazole moiety (39) and tested for their antifungal activity Among the tested compounds, this compound was found to be more active against Gibberella zeae, F. oxysporum and C. mandshurica than other ones. Hymexazol was used as standard drug [37].
Karthikeyan MS, et. al. synthesized 2,4-dichloro-5-flourophenyl containing 1,3,4-Oxadiazoles (40 and 41) and then final compounds were tested for their antimicrobial activity. Among the tested compounds, compound 40a, 40b, 40c, 41a, 41b and 41c showed good inhibition against Staphylococcus aureus, Escherichia coli. Compounds 40a, 40b, 40c, 41b and 41c showed good antibacterial activity almost equal to the standard i.e Ciprofloxacian. Compound 40c showed good bactericidal activity against Staphylococcus aureus, Pseudomonas aeruginosa and Klebsiella pneumoniae bacterial strains. Compound 40a, 40c and 41c showed good inhibition against all the fungal strains. Compound 40c exhibited good fungicidal activity against Candida albicans, Aspergillus fumigatus and Penicillium marneffei fungal strains and compared with standard drug Greseofluvin [38].
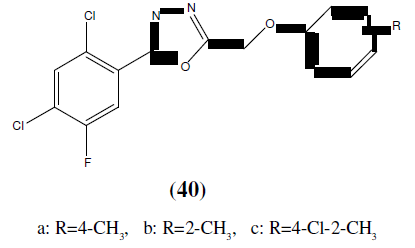
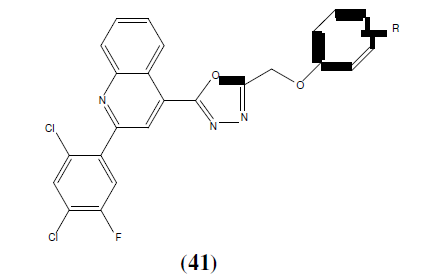
a: R=2-Cl, b: R=4-Cl, c: R=2, 4-Cl2
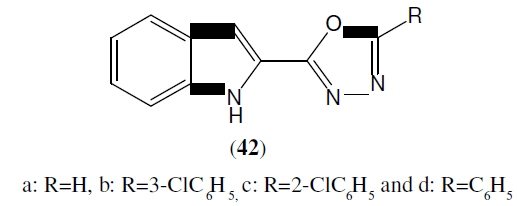
Bhardwaj et al. synthesized 1,3,4-Oxadiazoles derivatives and evaluated for their antimicrobial activity on different strains. These compounds (42) were synthesized, out of those only three found to be active against bacterial strains i.e Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa and none of the compound were found to be effective against fungal strains. Standard drug used were Norfloxacin and Fluconazole [39].
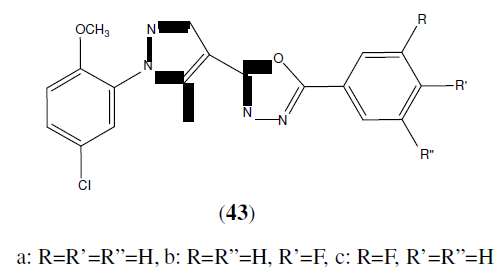
Rai NP, et al. synthesized 2-[1-(5-chloro-2-methoxyphenyl)-5-methyl-1H-pyrazol-4-yl]-5-(substituted phenyl)-[1,3,4- oxadiazoles] derivatives (43) and tested for their antibacterial activity. From the tested compounds, compound (43a) which is unsubstituted showed significant activity against Bacillus subtilis and moderate activity against Escherichia coli, Staphylococcus aureus Klebsiella pneumonia. Fluorine incorporated in phenyl ring (43b and 43c) of 1,3,4- oxadiazole showed improved activity against both Gram +ve bacteria i.e Bacillus subtilis, Staphylococcus aureus and Gram –ve bacteria i.e against Escherichia coli, Klebsiella pneumonia. These compounds were compared with Ampicillin as standard drug [40].
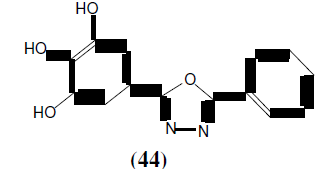
Jain N, et al. synthesized a series of 2-(3,4,5-trihydroxyphenyl)-5-aryl-1,3,4-oxadiazole derivatives (44). All the synthesized compounds were subjected to antimicrobial and anti-fungal activity. Antimicrobial activity was carried out against Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae and Staphylococcus aureus at a concentration of 100 μg/ml. Streptomycin was used as standard. Anti-fungal activity was performed against Aspergillus niger with test compounds at a concentration of 100 μg/ml. Ketaconazole was the standard drug [41].
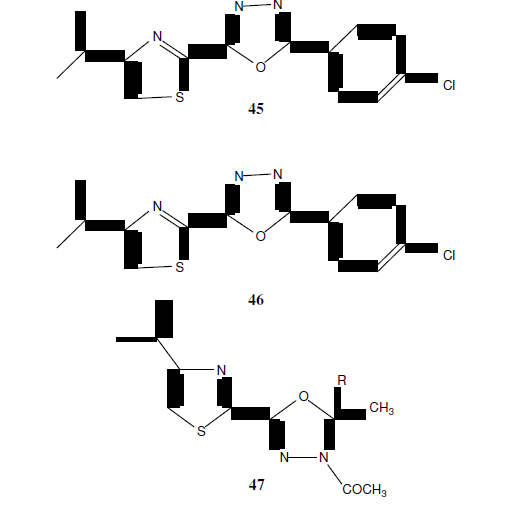
Kumar GVS, et al. synthesized some novel 2-substituted-5-[isopropylthiazole] clubbed 1,3,4-Oxadiazoles (45, 46 and 47) and tested for antimicrobial activity by broth microdilution method. Among the various synthesized compounds (45) showed improved antibacterial activity against Gram-positive bacteria i.e Staphylococcus aureus, Staphylococcus faecalis, Bacillus subtillis and compound (46) having p-methoxy substitution showed excellent antifungal activity against Saccharomyces cerevisiae, Candida tropicalis, Aspergillus niger. Compound (47) exhibited good inhibition against Gram positive bacteria. These tested compounds were compared with standard drugs i.e. Ciprofloxacin, Norfloxacin, Flucanozole [42].
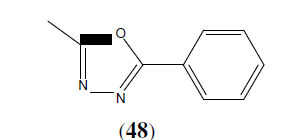
Patel NB, et al. synthesized a series of 1,3,4-oxadiazole derivatives (48) and tested there in vitro antimicrobial activity. The antimicrobial activity was examined against gram +ve bacteria S. aureus and gram –ve bacteria A. niger using the broth microdilution method [43].
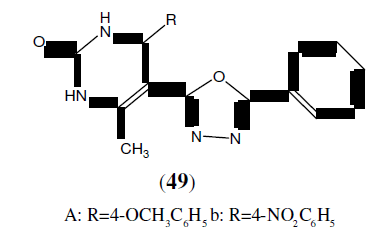
Mishra MK, et al. synthesized a series of 2,5-disubstituted-1,3,4-Oxadiazole derivatives (49) and then final compounds were tested for their antimicrobial activity by cup and plate method. Among the tested compound (49a) showed promising antibacterial activity against Gram +ve bacteria i.e Streptococcus pneumonia and compound (49b) showed promising antibacterial activity against Gram –ve bacteria i.e Escherichia coli as compared to standard drugs Ofloxacin and Levofloxacin [44].
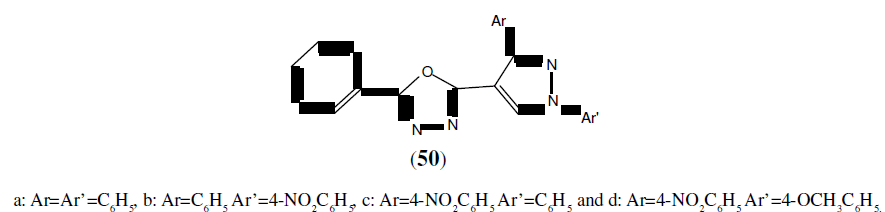
Prakash O, et al. synthesized a series of novel unsymmetrical 2,5-disubstituted 1,3,4- Oxadiazoles derivatives (50) and then the final compounds were tested for their antibacterial and antifungal activities. Among the tested compounds, compound 50a and 50b showed maximum antibacterial activity against Staphylococcus aureus and was compared with ciprofloxacin as standard drug. Compound 50c and 50d showed maximum inhibition against both of the fungi Aspergillus niger and Aspergillus flavus and was compared with Fluconazole as standard drug [45].
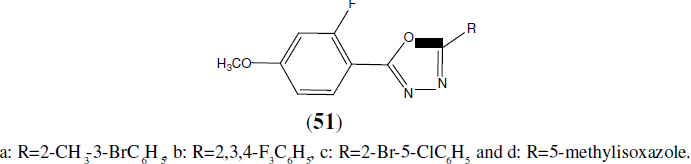
Chandrakantha B, et al. synthesized some novel 2-flouro-4-methoxyphenyl substituted 1,3,4-Oxadiazole derivatives (51) and screened them for antimicrobial activity by serial dilution method. Compounds tested for antibacterial activity was compared with standard drug Furacin and for antifungal activity standard drug was Flucanazol [46].
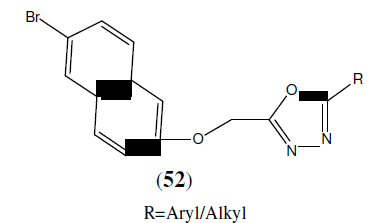
Mayekar AN, et al. synthesized a series of new 1,3,4-oxadiazole derivatives having 6- bromonaphthalene moiety (52). The newly synthesized compounds were characterized by analytical and spectral data. Antimicrobial activities of these compounds were carried out and some of them have exhibited good antimicrobial activity [47].
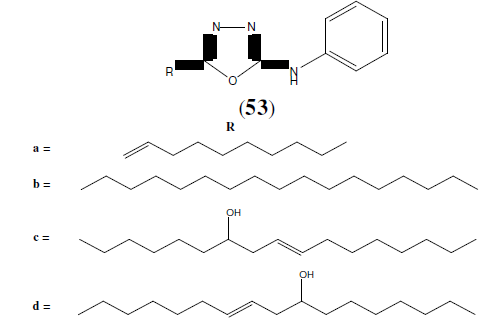
Farshori NN, et al. synthesized 5-alkenyl/hydroxyalkenyl-2-phenylamine-1,3,4- Oxadiazoles (53) and tested for in vitro antimicrobial activities by disc diffusion method. Among the synthesized compounds, compound 53b was found to be more active against fungal strain i.e Penicillium marneffei and was compared with greseofulvin as standard drug. Compound 53c and 53d was found to be more active against bacterial strains i.e Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Streptococcus pyogenes, Klebsiella pneumoniae and was compared with standard drug chloramphenicol [48].
Banday MR, et al. synthesized some newer 2,5-disubstituted-1,3,4-oxadiazole derivatives (54) and screened for their antibacterial activity. All the compounds were studied for their in- vitro antibacterial activity against two Gram negative strains such as Escherichia coli and Pseudomonas aeruginosa and two Gram positive strains like Bacillus subtilis and Staphylococcus aureus and their minimum inhibitory concentration (MIC) were determined. Ciprofloxacin was used as a standard drug [49].
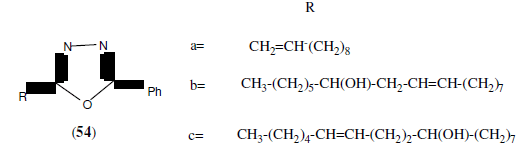
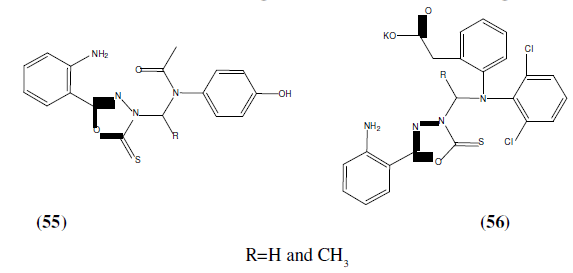
Kanthiah S, et al. a series of 5-(2-aminophenyl)-1,3,4-oxadiazole-2(3H)-thione derivatives (55 and 56). In-vitro anti-microbial activity was evaluated by disc diffusion method for all the newly synthesized compounds against gram +ve organisms such as Staphylococcus aureus, Streptococcus pyogenes, gram –ve organisms such as Escherichia coli, Klebsiella aerogenes and fungus such as Candida albicans. These Compounds showed moderate antibacterial and antifungal activities. Amikacin and ketoconazole (10 μg/ml) were used as reference standard drugs for antibacterial and antifungal activity respectively [50].
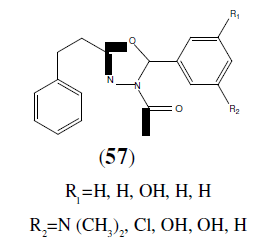
Kumar S, et al. synthesized a new series 1-(2-aryl-5-phenethyl-1,3,4-oxadiazole-3(2H)-yl)- ethanones derivatives (57) and found to exhibit good antibacterial and antifungal activity. These newly synthesized compounds were shown the maximum activity against the strains of micro- organisms Staphylococcus aureus and Pseudomonas aeruginosa [51].
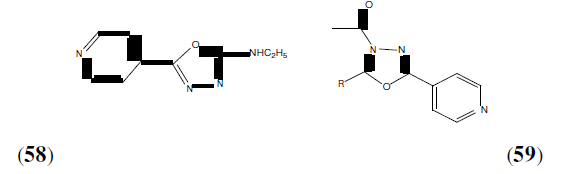
Mathew G, et al. synthesized 2,5-disubstituted-1,3,4-oxadiazole derivatives were obtained from aromatic aldehyde and acetic anhydride and POCl3. All the synthesized compounds showed significant analgesic, anti-inflammatory, anti-bacterial and anti-tubercular activities. But compound 58 and 59 was found to possess better activity then others [52].
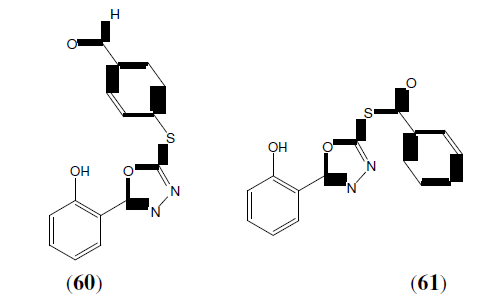
Parikh PK, et al. synthesized 1,3,4-oxadiazole derivatives as potential anti-bacterial and antifungal activity. Compound 61 has maximum activity against S. aureus and compounds 60 has Maximum antifungal activity against C. albicans [53].
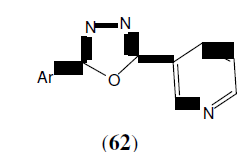
Shridhar AH, et al. synthesized a new series of 2,5-disubstituted-1,3,4-oxadiazoles derivatives (62) by reaction of nicotinic acid hydrazide with various substituted aromatic acids in presence of POCl . Some of the synthesized compounds showed very good antifungal activity when compared to standard. Antibacterial activity was evaluated against Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli and Bacillus subtilis. The standard drug used was Ampicillin and DMF was kept as solvent control. The antifungal studies were carried out against fungus Candida albicans and Aspergillus niger using Griseofulvin as standard [54].
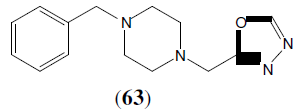
Bhardwaj S, et al. synthesized of 1-[(5-sustituted-1,3,4-oxadiazol-2-yl)methyl]-4- benzylpiperazines was carried out by all the Synthesized compounds were screened for their antibacterial activity against Staphylococcus aureus, Escherichia coli, Bacillus subtilisand Pseudomonas vulgaris. This compound (63) showed highest activity [55].
Kumar R, et al. synthesized some newer 1,3,4-oxadiazole derivatives (64) from biphenyl 4-carboxylic acid. Compounds were screened for in vitro antimicrobial activity against the representative panel of gram positive and gram negative bacteria. The result of antimicrobial study indicated that the presence of halogen atom in aromatic ring enhanced the antibacterial activity [56].
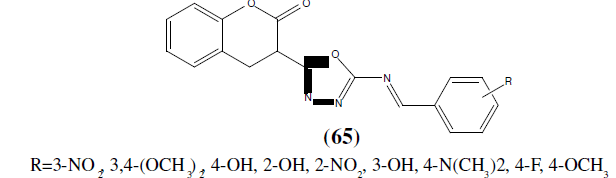
Bhat MA, et al. synthesized a series of 3-{5-[(E)-(substituted benzylidene) amino]-1,3,4- oxadiazol-2-yl}-2H-chromen-2-ones (65) have been synthesized from 3-(5-amino]-1, 3,4- oxadiazol-2-yl}-2H-chromen-2-one with different substituted benzaldehydes to form Schiff bases of coumarin-incorporated 1,3,4-oxadiazole derivatives. The compounds were screened against bacterial strains S. aureus NCTC (10418), E. coli NCTC (6571), and fungal strain C. albicans ATCC (10231) by cup plate method (agar diffusion method). Ciprofloxacin and Ketoconazole were used as a reference. Compound (4 m) without any substitution of the phenyl ring, which is attached to 1,3,4-oxadiazole moiety showed highly significant in vitro growth inhibition against [57].
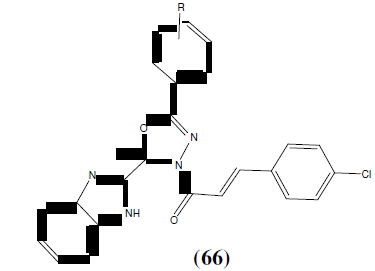
Desai et al. reported novel series of 1-(2-[1H-benzo(d)imidazol-2-yl]-2-methyl-5-aryl-1,3,4-oxadiazol-3(2H)-yl)-3-(4- chlorophenyl)prop-2-en-1-ones (66) under microwave irradiation technique. Synthesized compounds were tested for their in vitro anti-microbial activity against gram-positive, gram-negative strains of bacteria as well as fungal strains. Out of them, substituted derivatives with fluoro and nitro groups at para position displayed highest anti- bacterial activity [58].
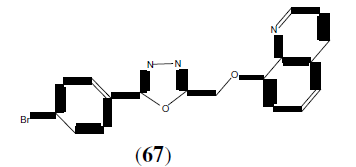
Adimule V, et al. synthesized some newer 1,3,4-oxadiazole compounds containing 8- hydroxy quinolone moiety as antibacterial agents. This Compound (67) showed greater inhibition on Staphylococcus aureus with MIC<6.25 μg/mL [59].
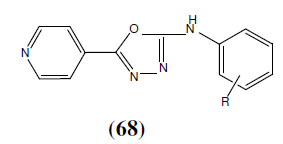
Yar SM et al. synthesized a series of 2-(substituted phenyl) amino-5-(4-pyridyl)-4H-1,3,4-thiadiazoles and 2-(substituted phenyl)amino-5-(4-pyridyl)-4H-1,3,4-oxadiazoles. All the compounds showed activity in the range of 33-99% in comparison to phenytoin which completely inhibited the convulsions. Compound 68a showed maximum activity and compound 68b [p-chloro substituted] showed good activity [60].
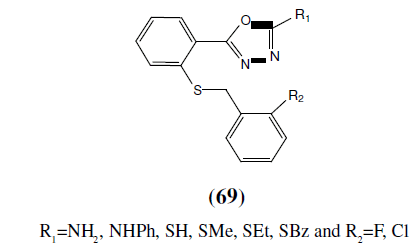
Zarghi A, et al. synthesized a series of some newer 2-substituted-5-{2-[(2- halobenzyl)thio)phenyl}-1,3,4-oxadiazoles (69) and investigated for anticonvulsant activities. Maximal Electroshock and pentylenetetrazole induced lethal convulsion tests showed that some of the synthesized compounds had significant anticonvulsant activity [61].
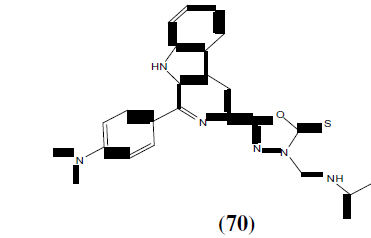
Shaharyar M, et al. synthesized a series of 2,5-disubstituted 1,3,4-oxadiazole derivatives and was tested for anticonvulsant activity. From the synthesized compounds compound (70) 2- (4-chlorophenyl)amino-5-(4-pyridyl)-1,3,4oxadiazole showed potent anticonvulsant activity [62].
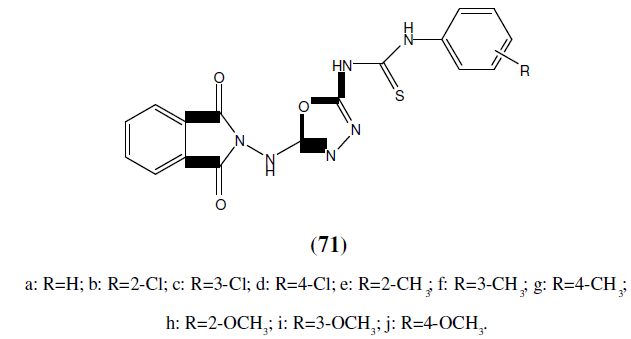
Bhat MA, et al. has synthesized a series of novel 1,3,4-oxadiazole derivatives of phthalimide (71) and evaluated for their anticonvulsant and neurotoxicity studies. Oxadiazole derivatives were synthesized by reacting phthalic anhydride with semicarbazide and hydrazine hydrate in presence of sodium hydroxide. Among the synthesized compounds compound 71j with para methoxy substituent demonstrated that distal hydrophobic center could be made more lipophilic than phenyl ring thus displaying the highest anticonvulsant activity [63].
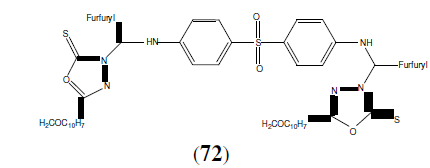
Mohamed A, et al. synthesized a series of 1,3,4-oxadiazole mannich base derivatives and synthesized compounds were tested for their antimycobacterial activity. They reported that eleven compounds exhibited excellent antimycobectrial activity. This compound (72) found to be most potent compound against both M. tuberculosis H37Rv and INH resistant tuberculosis [64].
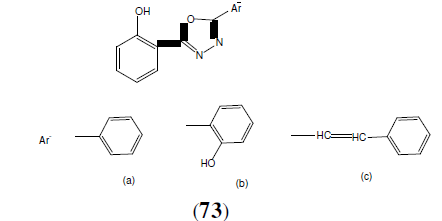
Pallon R, et al. synthesized some novel 1,3,4-oxadiazole derivatives (73) and carried out for their antitubercular activity by middle brook 7H9 medium against H37Rv strain as compared to standard drug streptomycin. Compound 73a have shown promising activity and 73b, 73c have shown moderate activity [65].
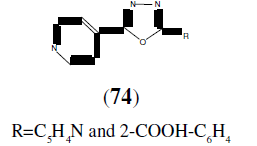
Dhansay D, et al. reported in vitro antitubercular activity of series of 2,5-disubstituted- 1,3,4-oxadiazole derivatives (74). These compounds exhibited better activity against a strain of mycobacterium tuberculosis H37Rv [27].
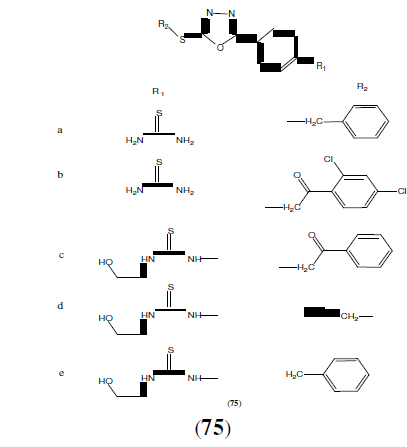
Macaev F, et al. synthesized a series of 5-aryl-2-thio-1,3,4-oxadiazole derivatives (75) and showed their anti-mycobacterial activities against Mycobacterium tuberculosis H37Rv. Structure activity relationship was performed for the given series by using electronic topological method combined with neutral network [66].
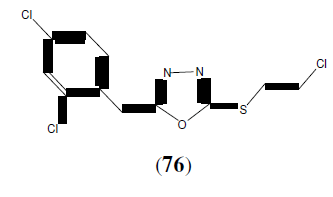
Bakal RL, et al. synthesized some newer 2,5-disubstituted 1,3,4-oxadiazole derivatives as potential candidate for treatment of XDR and MDR tuberculosis. This compound (76) showed potent antitubercular activity [67].
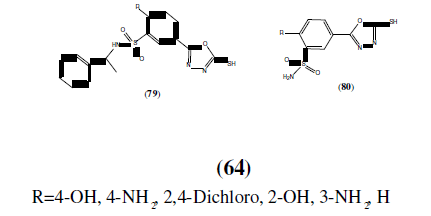
El-Emam AA, et al. synthesized anti-HIV-1 activity of certain 5-(1-adamantyl)-2- substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substitutedaminomethyl-1,3,4-oxadiazoline-2-thiones derivatives. The inhibitory activity of the compounds (77 and 78) against the human immunodeficiency virus type 1 (HIV-1) was determined using the XTT assay on MT-4 cells. The compound 78 was the most active among the compounds tested, producing 100, 43 and 37% reductions in viral replication at concentrations of 50, 10 and 2 μg/mL respectively. Compounds 77 with R=4-F, and 2-Br groups exhibited less anti-viral replication activity yet above 10% inhibition at concentrations of 2 μg/mL [68].
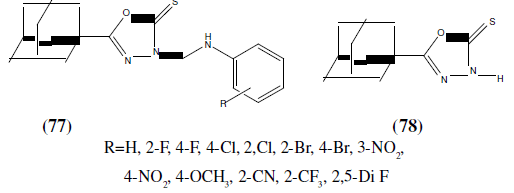
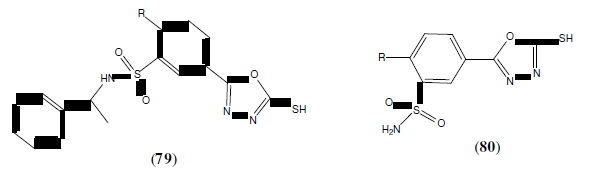
Iqbal R, et al, synthesized some novel benzenesulfonamides bearing 2,5-disubstituted- 1,3,4-oxadiazole derivatives and reported inhibitory activity for compounds (79 and 80) against the human immunodeficiency virus type 1 (HIV-1) which was also determined using the XTT assay on MT-4 cells. Compound 79 with the R=Cl group was the most active among the compounds tested, with 62, 21 and 14% reductions at concentrations of 50, 25 and 5 μg/mL respectively [69].
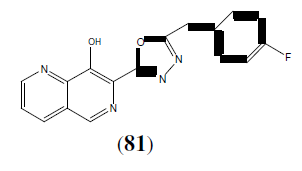
Johns B, et al, synthesized new derivatives containing the 1,3,4-oxadiazole derivatives (81) in combination with a ring system of 8-hydroxy-1,6-naphthyridine and reported antiviral activity (through inhibition of viral DNA integration) [70].
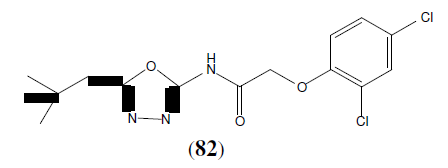
Bankar GR, et al, synthesized some newer 1,3,4-oxadiazole derivatives and also investigated whether the correction of endothelial dysfunction is dependent on high blood pressure normalization; in deoxycorticosterone acetate (DOCA-salt), and NG-nitro-L- arginine (L-NNA) in hypertensive rats. This Compound (82) is a T type Ca2+ channel inhibitor with an IC50 of 810 nM [71].
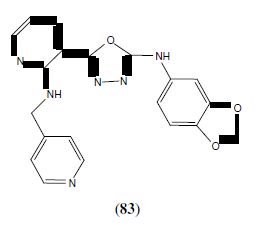
Ouyang X, et al. synthesized some 1,3,4-oxadiazoles derivatives and evaluated them for their ability to inhibit tubulin polymerization and to arrest mitotic division of tumour cells. Among the synthesized compounds, compound 83 showed potent activity [72].
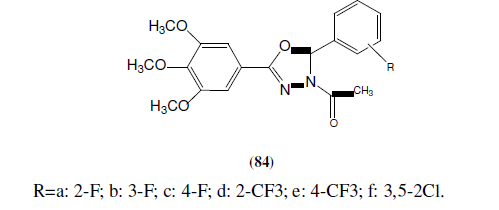
Baoan S, et al. synthesized some 3-acetyl-2-substituted phenyl-5-(3,4,5-trimethoxyphenyl)-2,3-dihydro-1,3,4-oxadiazole derivatives (84). Most of the synthesized compounds were found highly active against PC3 cancer cells and some were found moderately active against Bcap37 and BGC823 cells [73]
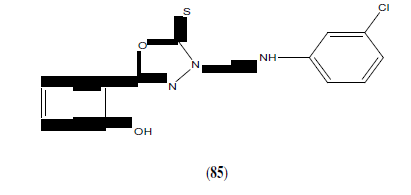
Ahmed SA, et al. synthesized series of 5-(2-hydroxyphenyl)-3-substituted-2,3-dihydro-1,3,4-oxadiazole-2-thione derivatives (85) and evaluated for their in vitro anticancer activity. These compounds have been selected for a full anticancer screening against a 60-cell panel assay where they showed non-selective broad spectrum and promising activity against all cancer cell lines. The active members in this study compared to 5-fluorouracil and cyclophosphamide as reference drugs, respectively [74].
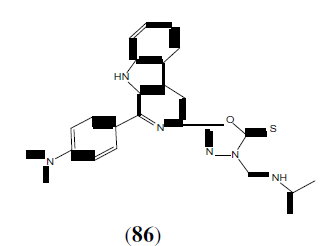
Savariz FC, et al. synthesized and evaluated the in vitro antitumor activity of new Mannich bases. Among the compounds studied, this compound (86) showed potent activity against melanoma (UACC-62), and lung (NCI-460) cell lines with GI50 values of 0.88 and 1.01 mmol/L, respectively [75].
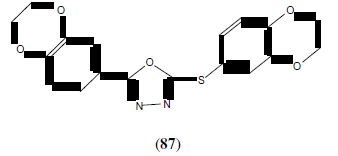
Zhang X-M, et al. synthesized and molecular docking studies of 1,3,4-oxadiazole derivatives possessing 1,4-benzodioxan moiety. This compound (87) showed potential anticancer agent [76].
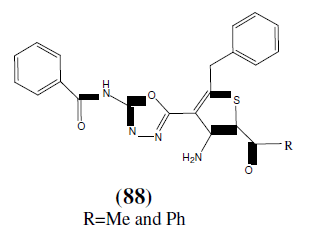
Bondock S, et al. synthesized some newer 2,5-disubstituted-1,3,4-oxadiazole derivatives (88) and evaluated for their antitumor activity and ctotoxic activity [77].
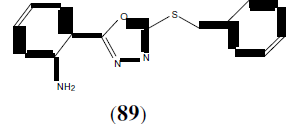
Liu K, et al. synthesized and reported the anti-proliferative and EGFR inhibition properties of a series of 2-(benzylthio)-5- aryloxadiazole derivatives. This compound 89 showed potent biological activity (IC50 =1.09 μM for MCF-7, and IC50 =1.51 μM for EGFR) [78].
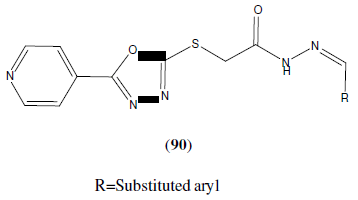
Ashan et al. synthesized series of 1,3,4-oxadiazole analogue (90) and evaluated for their anticancer activity. Compound 2-(4-chlorophenyl)-5-(4-fluorophenyl)-1,3,4-oxadiazole with methoxy phenyl at the fifth position of the oxadiazole ring showed more anti-cancer activity (leukemia, prostate) than compound 2-(4-chlorophenyl)-5-(-(4-methoxyphenyl)-1,3,4-oxadiazole with fluoro phenyl group at fifth positon of oxadiazole nucleus [79]
Sun et al. synthesized a series of 1,3,4-oxadiazole derivatives containing 1,4-benzodioxan (91) and screened their anti-tumor activity. Most of the titled compounds have potent anti-tumor activity and low toxicity. Among them, (E)-2-(2,3-dihydrobenzo[b] [1,4]dioxin-6-yl)-5-(2- fluorostyryl)-1, 3,4-oxadiazole compound showed the most potent biological activity against human umbilical vein endothelial cells with IC50 of 1.16 μM and inhibited activity of MetAP2 with IC50 of 2.08 μM, which was comparable to the positive controTNP-470. SAR indicated that compounds with electron-with drawing group showed stronger activity than that with electron-donating group with all IC50 values below 50 μM [80].
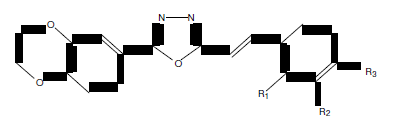
(a) R1=F, R2=H, R3=H, (b) R1=H, R2=F, R3=H, (c) R1=H, R2=H, R3=F, (d) R1=Cl, R2=H, R3=H, (e) R1=H, R2=H, R3=Cl, (f) R1=Br, R2=H, R3=H, (g) R1=H, R2=Br, R3=H, (h) R1=H, R2=H, R3=Br, (i) R1=CH3, R2=H, R3=H.
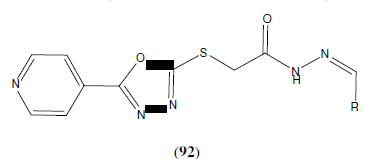
Zhang et al. reported a series of new 1,3,4-oxadiazole derivatives (92) containing pyridine and acylhydrazone moieties and developed as potential telomerase inhibitors. Among them, compound(E)-N´-(3,4-Dihydroxybenzylidene)-2-((5-(pyridine-4-yl)- 1,3,4-oxadiazol-2-yl)thio) acetohydrazide showed the most potent anti-cancer activity with IC50 of 0.76 ± 1.54 μM against four different original cancer cells (HEPG2, MCF7, SW1116 and BGC823) and exhibit telomerase inhibitory activity with IC50 of 1.18 ± 0.14 μM using telomeric repeat amplification protocol-polymerase chain reaction-enzymed-linked-immunosorbent assay [81].
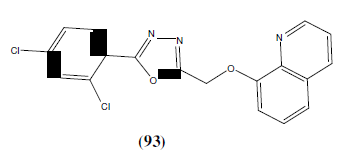
Adimule V, et al. synthesized some newer 1,3,4-oxadiazole compounds containing 8- hydroxy quinolone moiety as anticancer agents. The compound 93 was most potent anticancer agent against HeLa16 with IC50 cytotoxicity of 5-FU [59].
Oxadiazole is an important heterocyclic compound containing one oxygen and two nitrogen atoms in five membered ring in which 1,3,4-oxadiazole heterocyclic nucleus is a NCEs (new chemical entities) in this field. The present review summarizes the physicochemical properties, various synthetic procedures and the various pharmacological activities of 1,3,4-oxadiazole moiety. 1,3,4-oxadiazole derivatives is an important pharmacophore which play a major role in the pharmaceutical chemistry and broad range of important biological activities.