ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Ahmed A. Moosa, Ali Mousa Ridha, Ibtihal Najem Abdullha Department of Materials Engineering Technology, Engineering Technical College, Baghdad, Middle Technical University, Baghdad, Iraq |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
In this work, a low cost and abundant modified Iraqi bentonite was used for the removal of heavy metal Cr+3 ions from wastewater by adsorption onto raw and activated bentonites. The modification of raw bentonite was carried out by thermal activation (TAB), acid activation (AAB) and combined acid and thermal activation (AAB/TAB). The adsorption process was characterized by FTIR and BET. The results showed that the combined acid and thermal activated bentonite (AAB/TAB) has the optimum measured surface area 109.85 m²/g compared to that of all other types of activated bentonite. The optimum conditions for batch adsorption of Cr3+ ions and the maximum adsorption efficiency were found to be 99.83% for raw bentonite at 100 mg; 99.84 % for (TAB) at 75 mg ; 99.80% for (AAB) at 50 mg and 99.83% for (AAB/TAB) at 25mg. The maximum adsorption efficiency occurred at pH 6. The equilibrium data described by the Langmuir and Freundlich isotherm equations showed that the Langmuir isotherm gives better fit to the experimental data than Freundlich adsorption isotherm
Keywords |
| Adsorption, Heavy metal removal, Chromium removal, Carbone Nanotubes |
INTRODUCTION |
| Heavy metals in wastewaters are one of the most serious worldwide environmental problems. Heavy metals do not degrade biologically in the environment and can accumulate in living tissues particularly in human bodies causing significant physiological disorders.[1-2] |
| Chromium is a heavy metals and exist in several different forms such as chromium (III), and chromium (VI). The main sources of Cr are metallurgical, electroplating, tannery, mining, and paint industries [3-4]. Various techniques have been adapted to remove heavy metals from wastewater. These techniques include precipitation, electrochemical treatment solvent extraction, ion exchange and adsorption. Adsorption has been proven to be a cost effective, widely used and simple technique. [5] |
| Carbon nanotubes (CNTs) are allotropes of carbon which have a cylindrical or tubular structure [6] . CNTs can be Single -walled carbon nanotubes (SWNT) , Double wall (DWNT) and Multi-walled carbon nanotubes (MWNT) . CNTs have novel properties which are used in many applications such as : electronics, optics, water treatment and other fields of material sciences [7]. |
| CNTs have large specific surface area and small, hollow, and layered structures [8]. CNTs have been proven to be effective sorbents for the removal of metal ions as well as their complexes due to their large specific surface area as well as the presence of a wide spectrum of surface functional groups [9]. |
| Carbon Nanotubes (CNTs) were found to have great potential to be a good adsorbent for the adsorption of Cr (III) in aqueous solution. The adsorption parameters, such as the amount of MWCNTs, contact time, agitation speed, pH, were studied for the removal of Cr 3+ [10]. The results showed that the removal of Cr 3+ is mainly attributed to the affinity of Cr 3+ to the physical and chemical properties of the CNTs. Rao et al. [11] studied the removal of divalent metal ions (Cd2+, Cu2+, Ni2+, Pb2+, Zn2+) from aqueous solution using functionalized CNTs raw and surface oxidized carbon nanotubes (CNTs) for sorption. The sorption mechanisms appear mainly attributable to chemical interactions between the metal ions and the surface functional groups of the CNTs. The utilization of CNTs for the treatment of water and wastewater containing divalent metal ions is gaining more attention as a simple and effective means of pollution control. In Iraq; wastewater is discharged to river without treatment which causes water pollution. The aim of present work is to investigate the adsorption characteristics of Cr3+onto chemically modified CNTs. |
MATERIAL AND METHODS |
ADSORBATE |
| Chromium nitrate salt Cr(NO3)3.9H2O was used as Cr metal ions. Analytical-grade of Chromium nitrate salt, (99% purity, and molecule weight 400.15 g/mol, company Escrow,China). A 1000 mg/L standard stock solution of Cr3+ions was prepared by dissolving 7.692 g of chromium nitrate salt (Cr (NO3)3.9H2O, 99% purity, Escrow, China) into (1000mL) of deionized water and stirred using magnetic stirrer. |
CARBON NANOTUBES |
| MWCNTs used in this work were purchased from Cheap Tubes Inc. USA. MWCNTs was produced by CVD method and purified by plasma with purity of Ãâ¹ÃÆ99 % and diameter 13-18 nm with length 1-12 μm. |
FUNCTIONALIZATION OF MWCNTs |
| 0.5 g of MWCNTs were immersed in 500 ml of mixture of concentrated (HNO3:H2SO4) (1:3 by volume) at room temperature and ultrasonicated in high power ultrasonic path (frequency 60 KHz, power 140 W) for 30 min, the solution then was diluted 15 times and filtered in vacuum filtration system using filter papers with 0.2 μm in pore size. MWCNTs were washed many times during filtration using deionized water until the pH reached 7. The MWCNTs were dried in air for 24 hrs and then dried in vacuum oven at 100oC for 2hrs. The CNTs were characterized using FTIR [12-13]. |
BATCH ADSORPTION SYSTEM |
EFFECT OF ADSORBENT WEIGHT |
| To determine the optimum weight of MWCNTs as an adsorbent , a different weights (5, 15, 25, 50, 75,100, 125,150) mg of MWCNTs was mixed with 50 mL of chromium ions solution Cr3 +. The solution was then shaken for about 3hrs in a rotary shaker at fixed speed 140 rpm and temperature of 25oC. After shaking the solutions were filtered in vacuum filtration system. The remaining of Cr3+ ion concentrations in the filtrate were measured by atomic-absorption spectrophotometer. All experiments were performed at the pH 7 of the solution and 100 mg/L initial concentration of Cr3+ ion solution. The desired pH was adjusted using 1 M HCl and 1 M NaOH. |
EFFECT OF pH |
| To obtain the optimum of pH of Cr3+ solution pH adsorption onto MWCNTs, a different Cr3+ solution pH ( 2, 4, 6, 8, 10) were used with 50 mL of Cr3+ metal ions solution at initial concentration of 100 mg/L. The pH of each samples was adjusted by 1M of concentrated HCl acid and 1M NaOH by pH meter. The metal ion solutions Cr3+ of different pH was added to the bottles containing optimum weight of weight of MWCNTs . The bottles were then shaken in a rotary shaker for 3 hrs at 140 rpm and 25oC. The MWCNTs solution was then filtered using filter paper with vacuum. The remaining of Cr3+ ion concentrations in the filtrate were determined by using the atomic-absorption spectrophotometer. The optimum pH of Cr3+ metal ion solutions for removal of Cr3+ metal from aqueous solution was then selected |
Equilibrium Isotherms |
| Equilibrium isotherms experiments were carried out using optimum mass of MWCNTs and the optimum pH of Cr3+ metal ion solutions for removal of Cr3+ metal from aqueous solution. A 50mL of Cr3+ ion solution with different initial concentration (20,40,60,80,100, and 120 mg/L) was added in bottles containing the optimum adsorbent dosage of MWCNTs and optimum pH of solution. The solution was then shaken at a fixed speed of 140 rpm in rotary shaker using water bath at different temperatures of 25, 35 and 45oC for 3hrs. The shaker bath provides temperature indicator controller that controls the temperature for the desired value. After shaking the solutions were filtered using vacuum filtration. The remaining of Cr3+ ion concentrations in the filtrate were determined by using the atomic-absorption spectrophotometer. |
| The adsorption capacity, qe (i.e the mass of solute adsorbed per mass of adsorbent) was then plotted versus the equilibrium concentration of the solution, Ce to obtain the equilibrium isotherm curves. The adsorption of metal ions onto the adsorbent surface at specific time was estimated from mass balance equation [14]: |
| Where q is quantity of adsorbate (mg/g) , V is the total volume of adsorbate solution (L). Co and Ce are the initial and equilibrium concentration of adsorbate solution at equilibrium in (mg/L) respectively .W is the adsorbant weight (g). |
 |
RESULTS AND DISCUSSION |
FTIR |
| The Fourier Transform Infrared Spectrophotometer, FTIR, spectra of oxidized MWCNTs is shown in Figure 1. There are four major peaks, located at 3410.15, 2370, 1701.22 and 1566.2 cm−1. The peak at 3410.15 cm−1 can be assigned to the O-H stretch from carboxyl groups (O=C−OH and C−OH). The peak at 2364 cm−1 can be associated with the O−H stretch from strongly hydrogen-bonded −COOH [7]. It can be seen that the peak in 1701.22 cm−1 is associated with -C=O stretching of the carboxyl group. The peak at 1566.2 cm−1 is related to the carboxylate anion stretch model [21]. The many oxygen-containing functional groups that were generated on the surface of modified CNTs, providing various adsorption sites, and increasing the adsorption capacity [22]. Functionalization process by acidic oxidation of carbon surface can offer not only a more hydrophilic surface but also a larger number of oxygencontaining function groups, which increase the ion-exchange capability of carbon material. The specific area of MWCNTs as measured by the BET method was found to be 63.17 (m2/g). MWCNTs have high surface area. |
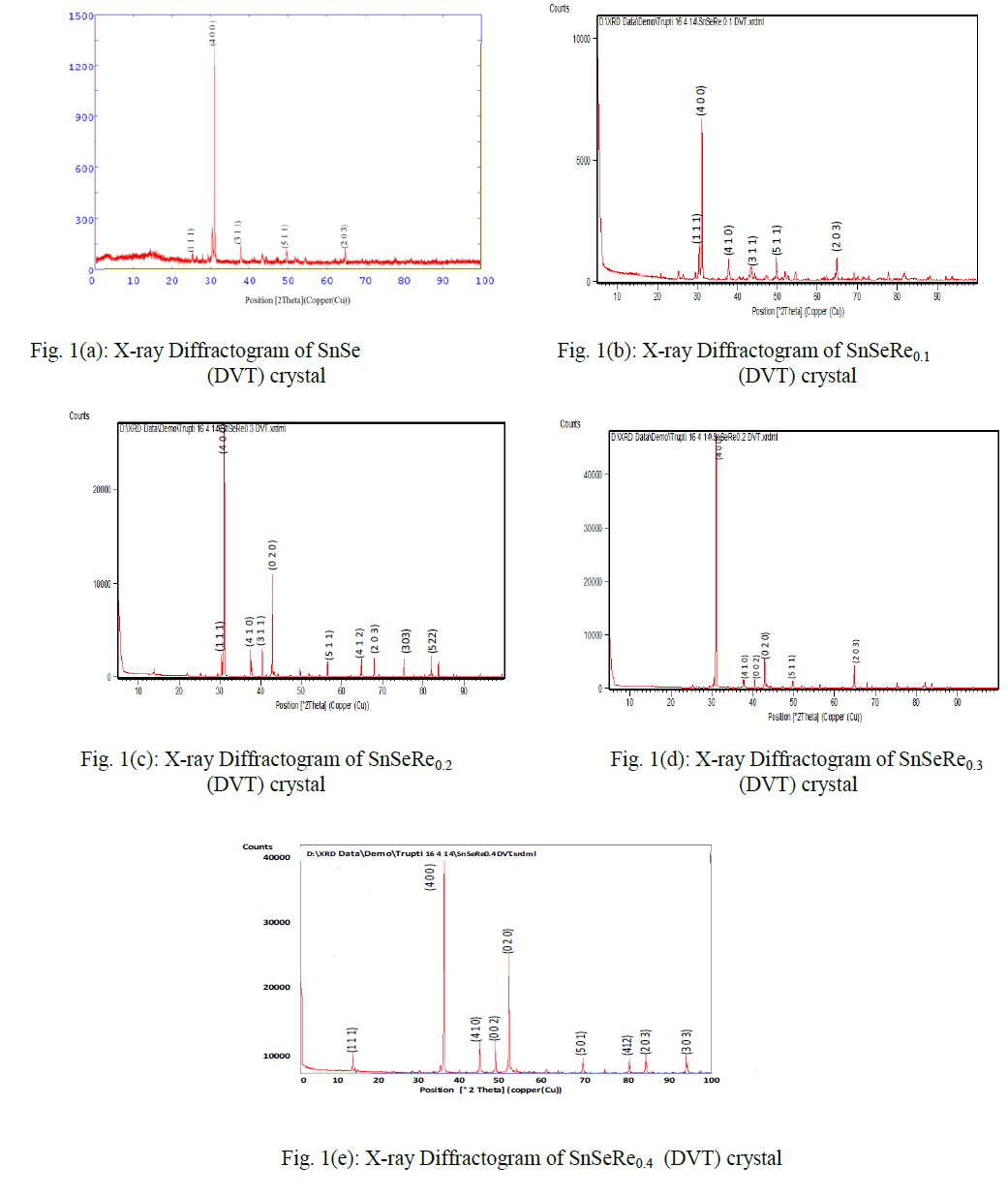 |
EFFECT OF ADSORBENT WEIGHT OF MWCNTs ON ADSORPTION PROCESS |
| Different weights (5, 15, 25, 50, 75,100, 125,150) mg of MWCNTs were used to study the effect of MWCNTs weight on adsorption efficiency for the removal of Cr3+ at pH 7. Figure 2 shows the percentage removal of Cr3+ ions increases with the increase of the weight of MWCNTs. This is due to the availability of more sorption active sites and high surface area until equilibrium is reached. This is in good agreement with the work of [23]. The functional groups make MWCNTs more suitable for the adsorption of Cr3+at smaller weight. The maximum adsorption efficiency (99.99%) occurs at small weight, 25 mg of MWCNTs. |
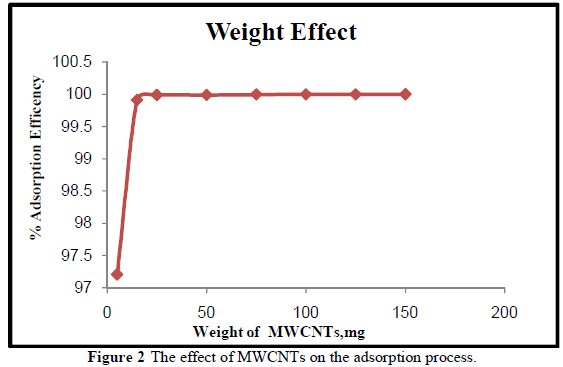 |
EFFECT OF CHROMIUM SOLUTION pH ONTO MWCNTs ON ADSORPTION PROCESS |
| The adsorption efficiency of MWCNTs for the removal of Cr3+ions increased at pH 4-6 as shown in Figure 3. The adsorption efficiency increases gradually with increasing pH. The minimum adsorption observed at pH 2 values might be due to the fact that the higher concentration and mobility of hydrogen ions (H+) present at lower pH favored the preferential adsorption of hydrogen ions than Cr3+ metal ions. Also , the MWCNTs surface are predominantly covered by H+ at low pH values which prevents metal ions from approaching the binding sites[24]. Increasing the pH from 2 to pH 6 will increase the Cr3+ adsorption due to the fact that the metal ionic species become less stable in solution. At higher pH values (i.e., pH 6-10) , the adsorption efficiency decreased, which may have been due to the precipitation of chromium in the form of hydroxide. The maximum adsorption efficiency (99.91 %) occurred at the pH 6 which is in agreement with the work of Kosa, et al., [24]. |
 |
ISOTHERM OF MWCNTs |
| The data of the functionalized MWCNTs at optimum conditions (surface area 63.17 m2/g, pH 6, 25mg) were used at different Co (20, 40, 60, 80, 100,120 mg/L) to construct the experimental model and the Langmuir and Freundlich isotherm at 25oC . These data were then used in Figure 4.20 and Table 4.4. Based on correlation coefficient R2 (from Table 4.4) it can be concluded that the Langmuir model yields better fit to the experimental data than the Freundlich adsorption isotherm. |
| The effect of different temperatures (25, 35, 45 oC) on the adsorption isotherm of Cr+3 ions onto MWCNTs was investigated. The results from Table 1 shows that the Langmiur adsorption isotherm gives the best fit to the adsorption isotherm for the temperatures (25, 35, 45 oC). The values of Langmuir constants b, qm and correlation coefficient R2 increased with temperature due to the increase in the negative charges of the surface thus, higher adsorption occurred at higher temperatures . This is in agreement with the work of [25]. |
Methods
|
THERMODYNAMIC PARAMETERS OF MWCNTs |
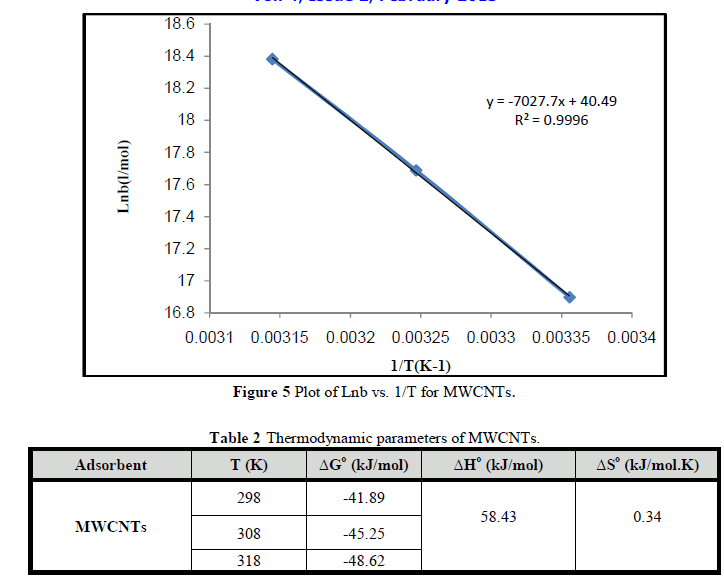
| Figures 5 shows plot of lnb vs. (1/T) for MWCNTS. Table 2 shows the changes in the free energy of adsorption (ΔG°), enthalpy (ΔH°) and entropy (ΔS°). The negative values of ΔG° indicate that process is spontaneous. The positive value of ΔH° indicated the endothermic nature of the process, which means there is a strong interaction between MWCNTs and Cr3+ ions and the adsorption increases with temperature.. The positive value of ΔS° indicates the affinity between MWCNTs and the Cr3+ ions and structural changes in MWCNTS as reported by Chen et al., [26]; Teker and Imamoglu [27]. |
 |
CONCLUSIONS |
| The functionalized MWCNTS is an effective adsorbent and can be successfully used for removing of Cr3+ ions from wastewater. The maximum adsorption efficiency of MWCNTs is 99.98% at 25 mg and at pH 6. The equilibrium isotherm of (MCNTs) shows that the Langmuir isotherm fits the experimental data better than Freundlich adsorption. The negative values of ΔG° indicate that process is spontaneous. The positive value of ΔH° indicates the reaction is endothermic. The positive value of ΔS° indicates the random character of adsorption of Cr3+ ions on the MWCNTs. |
References |
|