e-ISSN: 2319-9849
e-ISSN: 2319-9849
Nuclear Materials Authority, PO Box 530, El-Maadi, Cairo, Egypt
Received Date: 23/05/2016; Accepted Date: 08/11/2016; Published Date: 14/11/2016
Visit for more related articles at Research & Reviews: Journal of Chemistry
El-Sela shear zone occurs in the younger granite rock of Gabal El-Sela area, South Eastern Desert, Egypt near the Sudan Frontier. It comprises line–arranged intrusions trending ENE-WSW and extend for about 1.5 km with width reaches up to 40 meter. These line–arranged intrusions include altered microgranite dyke, altered basic dyke and multi-phase quartz vein. Uraniferous mineralization was subjected to sulphuric acid column leaching for the leaching and recovery of uranium. Column leaching tests were performed to study the general leaching parameters of uranium from the obtained mineralization. The later include the grain size, acid concentration, height of mineralization load on column and the overall solution flow rate. The leaching process of the mineralization from El-Sela was conducted with the columns being charged with 120 kg mineralized sample with an average uranium content 0.14%. Results showed that uranium leaching rate calculated by uranium concentration on uranium pregnant solutions was about 81.03% in 38 days of test. Uranium from the obtained leach liquor was achieved by using anion-exchange method followed by later elution by using acidified sodium chloride solution. Finally, uranium precipitation from the eluted solution was performed using sodium hydroxide solution. A case study is provided of how the results from batch column tests, were used to optimize the acid leaching and recovery of uranium from the studied mineralization to be applied in large scale using vat leaching technique.
El-Sela shear zone is located at Gabal El-Sela area in the southern extremity of the Eastern Desert of Egypt near the Sudan Frontier and occupies the southern half of Elba topographic sheet (NF-37 I). It lies at about 22 km SW of Abu-Ramadcity. It is bound by Latitudes 22°17ʹ44ʺ-22°18ʹ10ʺ N and Longitudes 36°13ʹ28ʺ- 36°14ʹ27ʺ E (Figure 1). The area was studied geologically, mineralogically, geochemically and radiometrically by many authors, e.g., Abdel-Meguid et al. studied the uranium potentiality of the Eastern Desert granites and concluded that El-Sela granite represents a highly fractionated high-K calc-alkaline (HKCA) magma comprising primary muscovite and occurring as a medium sized granite pluton affected by high alteration [1]. They also concluded that El-Sela granite is enriched in uranium-bearing uranothorite, high U-monazite and high U-zircon. El Afandy et al. concluded that the granites of Gabal El-Sela and Gabal Qash Amir originated from a metaluminous to weakly peraluminous alkaline magma and developed within an extensional regime in a within-plate tectonic setting [2]. Ibrahim et al. studied the U-fertility criteria applied to El-Sela granite, and concluded that the geology and tectonic history beside the spectrometry, geochemistry and mineralogy of El-Sela granite represent promising criteria for U-fertility [3].
Shahin studied the occurrence of uraniferous iron and manganese oxide deposits in biotite granite north east Gabal El- Sela area. He concluded that the uraniferous iron and manganese oxide deposits and the hydroxides associated with kaolinite may represent a gossan on the top of a vein-type U-bearing deposit [4]. Aly studied the structural control of El-Sela granites and associated uranium deposits [5]. He concluded that most of the uranium anomalies are delineated along ENE–WSW and NNW–SSE shear zones where quartz-bearing veins bounded the lamprophyre dike and microgranites and dissected them in relation to the successive fracturation and brecciation corresponding to the repeated rejuvenation of the structures. Therefore, the structural controls of the uranium mineralization in the El-Sela area appear to be related to the interaction between inherited ductile fabrics and overprinting brittle structures. Aly and Lentz studied the mineralogy, geochemistry and age dating of shear zone-hosted Nb-Ta-, Zr-Hf-, Th and U-bearing granitic rocks in the Ghadir and El-Sella areas, South Eastern Desert, Egypt [6]. They concluded that the rare metal minerals of mineralized altered granites within El-Sella shear zones are columbite-tantalite minerals as ferrocolumbite, pyrochlore, and fergusonite, Th-minerals (cheralite, uranothorite, and huttonite monazite), Hf-zircon, monazite and xenotime in the El-Sella shear zone.
The leaching and recovery of uranium has been studied on El-Sela mineralization, from these studies, Ibrahim et al., have performed a preliminary study to recover uranium from the study area through applying acid agitation leaching technique using sulfuric acid, then the uranium was removed from the pregnant leach liquor by Amberlite IRA–400 anion exchange resin [7]. The applied uraniferous El-Sela sample experiment assayed 950 U ppm and recovered in the form of sodium di-uranate (Na2U2O7). They found that El-Sela uranium ore is easily leachable. Ibrahim et al. have applied leaching process on technological scale sample for studying the mining ability, leaching characteristics and the recovering conditions, they found that El-Sela U-ore material is easily mineable and easily recovering [8]. Mira and Ibrahim evidenced the biogenic origin of the tetravalent uranium minerals where they recorded natural interaction between the uranium and the bacteria and fungi species within the studied rock [9].
Uranium was recovered from the sulphate leaching solutions of El-Sela mineralization in a pilot scale using Amberlite IRA400 packed in fixed columns with flow rate of 2.3 l/min [10]. Mirjal et al. have improved a process for uranium recovery from leached pulps of low grade ore using the resin-in-pulp method through using pachuca column [11]. Uranium was recovered from El-Sela leach pulps using Resin-in-pulp technique [12]. Finally, Salman et al. have successfully recovered REEs from El-Sela solid residue after conventional leaching operations with sulfuric acid [13]. The main objective of the present investigation is studying uranium leaching and recovery from El-Sela by column leaching technique and recovery by ion exchange resin adsorption and elution. In this type of study, column tests are used to evaluate a heap leach process using a batch type of testing methodology. It is impractical to conduct continuous column leach testing for evaluation of a heap leach process in the laboratory.
El-Sela shear zone comprises line–arranged intrusions trending ENE-WSW and extend for about 1.5 km with a wide reach up to 20 meter. These line–arranged intrusions include multi-phase quartz vein, altered microgranite dyke and altered basic dyke. A detailed geologic study was carried out on biotite ± muscovite granite of the El-Sela shear zone (Figure 2). This granite is highly weathered, cavernous and exposed as low to moderate separately hills, coarse-grained, pink to pinkish gray in color, mainly composed of K-feldspar, quartz, plagioclase, biotite and rare muscovite. It is characterized by the presence of iron and manganese oxides filling joints and fractures indicating the enrichment of this granite by iron and manganese mineralization. This granite is also enriched with altered pyrite, which is in sometime leaching out leaving cubic vugs and patches of deep red color of hematitization. This granite is intruded by microgranite and basic dyke, the microgranite dyke occurs as dyke and sheets, mainly injected along the ENE-WSW direction. This dyke is whitish buff to buff, leucocratic, fine grained, massive, equigranular texture and composed essentially of quartz, plagioclase K-feldspare and biotite. It is very rich in pyrite and characterized by the presence of extremely abundant manganese oxides filling joints and fractures. Thickness of this dyke varies from 30 cm to 5 m and extend for more than 1500 meters appear and disappear along the ENE-WSW direction. Locally; it is completely altered to pale pinkish brown color enriched by box work vugs of the dissolute pyrite. In some time, it is characterized by porphyritic textures with prominent phenocrysts of plagioclase and quartz minerals. Field relations indicate that this microgranite dyke is dissected by basic dyke.
Basic dyke follow the main fault planes with subvertical to steep dipping to the south. They extend along the ENE-WSW shear zone for more than 1.5 km long, appear and disappear in some areas with width varies from 1m to 5m. They are dark gray to grayish green color, fine grained, mostly altered, enriched by iron oxides and composed essentially of plagioclase, amphibole, chlorite, epidote and little quartz. In some time, they are characterized by porphyritic textures with prominent phenocrysts of both orthoclase and quartz minerals. Field relations indicate that this dyke intruded both fine-grained granite and coarse-grained biotite granite with sharp contact.
Sample Preparation
Representative samples from El-Sela mineralization used in this study ware obtained by Nuclear Materials Authority (NMA), Egypt having the chemical composition of 0.14% U, 0.33% P2O5, 70.61% SiO2, 16.15% Al2O3, 5.37% Fe2O3, 1.44% TiO2, 0.75% MnO, 1.1% MgO, 0.6% CaO, 0.07% Na2O, 1.08% K2O and 2.1% L.O.I.
According to the plan of laboratory experiments, samples for agitation leaching and column leaching are crushed into a particle grain size. This particle size meets the requirements for maximum particle diameter not to exceed 1/10 of the inside diameter of the column [14]. Particle size has certain effects on leaching rate. The size distributions of original samples are shown in Tables 1 and 2.
| Particle size, mm | Weight, kg | Weight percentage, % |
|---|---|---|
| -40~+20 | 70 | 58.4 |
| -20~+10 | 25 | 20.8 |
| -10~+5 | 25 | 20.8 |
Table 1: Size distribution of -40 mm original samples.
| Particle size, mm | Weight, kg | Weight percentage, % |
|---|---|---|
| -10~+5 | 5.0 | 50.0 |
| -5~+2 | 2.5 | 25.0 |
| -2 | 2.5 | 25.0 |
Table 2: Size distribution of -10 mm original samples.
Agitation Leaching Experiment
Acid agitation leaching process depends largely on several factors which must be carefully studied to obtain the optimum leaching conditions. These factors included sulphuric acid concentration, agitation time, particle size and solid/liquid ratio. The technological sample, collected from El-Sela area, South Eastern Desert was crushed to the grain size of about "–100 mesh" size (-0.15mm). The leaching process was carried out using proper concentration of H2SO4 under moderate conditions of 50 g/l, solid/liquid ratio 1/2 and agitation for 6 hours. The heat of dilution generated due to addition of concentrated sulphuric acid to the slurry where itself heating the mixture to about 45°C. During the solid/liquid separation and washing steps, classification of the solids to slime (or fine solids) and coarse size were done. The residue was dried in an oven at 110°C, weighted, grounded and analyzed for un-leached uranium.
Column Experiments
Column leach test: In this experiment, leaching variables of grain size, acid concentration, height of mineralization load on column and liquid-solid ratio are investigated in columns batch leaching experiment. The experimental variable conditions are: mineralization grade 0.14% U, ore size (-10, -20, -40 mm), acid concentration (20, 40, 70 g/l), Lixiviant flow rate (0.15, 0.1, 0.05) L/min/m2 and height of mineralization load on column (1, 2, 3 m). A simple down flow, gravity-driven column design will be utilized for the column leaching study (Figure 3). Recommended columns materials are PVC pipe with a (4, 8, 12-inch) inside diameter. The bottom of each column equipped with a PVC base plate containing a piece of screen to prevent loss of solid sample from the column during leaching. Optionally, 2 to 4 inches of clean silica sand can be loaded into the base of the columns prior to sample addition. The columns charged with mineralized sample which average uranium content is 0.14%. The base plate equipped with a valve to control the discharge of column effluent. A funnel and suitable 30-liter receptacle was placed under the column. A 20-liter feed container was placed ± 50 cm above the column. Sulphuric acid solution is used as lixiviant in the experiment. Lixiviant is stored in the overhead tank, adjust the adjustable valve to control the flow rate of lixiviant, allow the lixiviant down flow through mineralized bed by suitable flow rate of (10 h/d), leaching liquor is collected in the solution collection basin. Take sample from leaching liquor, and analyze its uranium content, pH (free acidity) and other impurities to decide whether the effluent is recycled as internal leaching solutions (ILS) or moved to recovery circles pregnant leaching solution (PLS). The end point of leaching mainly depends on economic indicator, if the value of extracted uranium is less than its operation cost, leaching can be stopped. Of course, if technical indicator such as leaching efficiency is uniquely concerned, leaching could continue. Reaction with hexavalent uranium, which dissolves as UO2 2+ cation, produces uranyl sulfate and complex uranyl sulfate anions as follows:
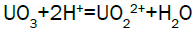
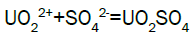
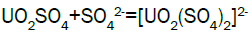
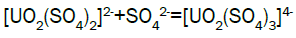
Adsorption, elution and precipitation experimental: In this experiment, all the operations are manually carried out. The adsorption and elution apparatus are depicted in Figure 3. The apparatus includes overhead tank, adjustable valve, PVC column with a 2.0-inch inside diameter, ion exchange resin, and sampling beaker. Strong base anionic exchange resins (D263B Chinese resin) used in ion exchange experiment. Pregnant solution is put into the overhead tank, adjust flow rate through valve to allow feed pregnant solution down flow the column; sample is taken from effluent at periodic intervals. Measure the volume of effluent and analyze uranium of sample [15]. Typical reactions between the mobile ion on resin and the uranium ions in solution will process as follows.
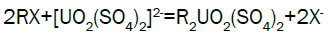
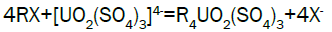
The eluted uranium solution from El-Sela,10% NaOH was added till pH 6.5-7.5. A bright yellow precipitate (yellow cake) of sodium di-uranate (Na2U2O7) was obtained. Typical reactions during precipitation with caustic soda are shown 2UO2SO4+6NaO H=Na2U2O7↓+2Na2SO4+3H2O, 2UO2SO4+2NaOH+4H2O=UO2)2SO4.4H2O+2Na2SO4 UO2SO4+2NaOH+(x-1) H2O=UO3.xH2O+Na2SO4
Analytical Method
Uranium was analyzed in the corresponding aqueous phases using Arsenazo III reagent under different conditions [16]. For this purpose, a Lambada UV/VIS spectrophotometer (Perkin-Elmer, USA) was used. In addition, uranium was also analyzed by an oxidimetric titration method against ammonium meta vanedate in the presence of diphenylamine sulfonate indicator prior to titration; proper reduction of uranium was performed using ferrous sulfate [15].
Agitation Leaching Results
El-Sela mineralization samples containing 0.14% U was laboratory subjected to uranium agitation leaching using the variable conditions to verify the conditions which achieve high uranium leaching efficiency. The suitable conditions that verify both economic advantages and higher leaching of uranium showed in Table 3.
| Conditions | H2SO4Concentration, g/l | Agitation Time, h | Ore Size, mm | Solid/Liquid ratio | Temperature, °C |
|---|---|---|---|---|---|
| El Sela | 50 | 6 | -0.15 | 1/2 | 40 |
Table 3: Optimum conditions of the agitation leaching on El Sela uranium mineralization.
Column Leaching Results
Column tests can then be performed on the different parameters to explore the suitable or near-optimal range for parameters, grain size, acid consuming, height of mineralization load on column and solution flow rate. Column leach experiments yield much improved estimates of the reagent consumption and when conducted at the intended heap stacking height, they provide kinetic information that can be applied to heap leach process design. Column leach experiments in closed circuit with metal recovery step (and with a bleed as appropriate) can simulate the anticipated accumulation of chemical species in solution, and such solution samples can be used for detailed downstream purification and metals recovery test work.
Study of the relevant heap leaching factors
Effect of grain size: In heap leaching, the particle size of the feed sample has a significant effect on uranium extraction. In the present work, the leaching characteristics of three different particle sizes of El-Sela uranium mineralization have been studied; namely -40, -20 and -10mm. The obtained results are collectively shown in Table 4. The above-mentioned test results indicate that the particle size has a notable effect on the leaching efficiency. In El-Sela uranium mineralization (-10mm sample), the leaching efficiency based on uranium analysis in solution is 75.5%, but that of –40mm sample is only 45.8%. Small particle size has the advantage of having a large specific surface area and more uranium minerals in small particle size can be exposed on its surface than those in large particle size. In the meantime, the thickness of the diffusion layer in small particle size is small, and accordingly it would have a high diffusion rate. The leaching rate is indeed proportional to the diffusion rate. During the early stages of leaching the particle size does not influences recovery. Finer size distribution, however, do enable a high rate of recovery to be maintained over a long period.
| Column. No. | A | B | C |
| Grain size, mm | -40 | -20 | -10 |
| Amount of sample, Kg | 120 | 20 | 10 |
| Grade of feed, ppm | 1400 | 1400 | 1400 |
| Concentration of lixiviant, g/L | 20 | 20 | 20 |
| Sprinkling intensity, L/S | 0.05:1 | 0.1:1 | 0.1:1 |
| leaching time, d | 33 | 36 | 40 |
| Leaching efficiency on solution, % | 45.8 | 65.0 | 75.5 |
| Accumulative liquor solid ratio | 0.75 | 1.5 | 1.7 |
| acid Consumption, g/kg | 15 | 30 | 34 |
| U in residue* ppm | 240 | 110 | 80 |
Table 4: Effect of Grain Size on Leaching Efficiency of El Sela mineralization.
Effect of Acid Concentration: Acid concentration of lixiviant is an important variable of leaching process. A reasonable acid concentration used in leaching is related to the characteristics and particle size of the feed ore and the leaching conditions. Excessive acid can increase the leaching rate, and cause high acid consumption. Also, high acid concentration may result in dissolving an excessive amount of associated gangue minerals in the ore. Free acidity in the leaching liquor should be maintained a certain level to prevent precipitation of dissolved uranium. Three different acid concentrations; namely 20, 40 and 70 g/L were tested in this experiment. The test results are shown in Table 5. The obtained results clarify increasing dissolution efficiency of uranium with increasing H2SO4 concentration, from 75.5% at 20 g/l up to 81.5 at 70 g/l H2SO4 but the duration time at 70 g/l less than that at 20 g/l. on the economical view the different between the efficiency at 40 g/l and 70 g/l was very small (0.6%) so 40 g/l is suitable in large scale.
| Column. No. | A | B | C |
|---|---|---|---|
| Grain size, mm | -10 | -10 | -10 |
| Amount of sample, Kg | 15 | 15 | 15 |
| Grade of feed ore, ppm | 1400 | 1400 | 1400 |
| Sprinkling intensity, L/S | 0.1:1 | 0.1:1 | 0.1:1 |
| Concentration of lixiviant, g/L | 20 | 40 | 70 |
| leaching time, d | 40 | 38 | 35 |
| Leaching efficiency on solution, % | 75.5 | 80.9 | 81.5 |
| Accumulative liquid solid ratio | 1.7 | 1.2 | 1.1 |
| Acid consumption, g/kg | 34 | 48 | 77 |
| U in residue* ppm | 80 | 63 | 55 |
Table 5: Effect of Acid Concentration on Leaching Efficiency of uranium.
Effect of solution flow rate, (l/m2/min) on leaching efficiency of uranium from El-sela mineralization:
During column leaching, solution flow rate or sprinkling intensity refers to the volume of lixiviant per square meter (L/m2/ min) used for leaching in one cycle. Three kinds of flow rate are tested in the experiments (0.15, 0.1, 0.05L/m2/min). Table 6 shows the test results. The effect of sprinkling intensity is not as obvious as the effect of particle size and acid concentration on leaching. The column with low sprinkling intensity needs more leaching time (45 day) but it would lead to a high uranium concentration (maximum and average) of leaching liquor.
| Column No. | A | B | C |
|---|---|---|---|
| Grain size, mm | -10 | -10 | -10 |
| Amount of feed ore, Kg | 15 | 15 | 15 |
| Grade of sample, ppm | 1400 | 1400 | 1400 |
| Concentration of lixiviant, g/L | 40 | 40 | 40 |
| Sprinkling intensity, L/S | 0.15:1 | 0.1:1 | 0.05:1 |
| Cumulative leaching time, d | 33 | 38 | 45 |
| Leaching efficiency on solution, % | 78.3 | 80.9 | 82.0 |
| Accumulative liquor solid ratio | 1.38 | 1.2 | 1.15 |
| Acid consumption, g/kg | 55.2 | 48 | 46 |
| U in residue* ppm | 65 | 63 | 50 |
Table 6: Effect of Sprinkling intensity on Leaching of El Sela mineralization.
Effect of height of mineralization load on recovery of uranium: As shown in Figure 4, the effect of uranium mineralization height on column (three columns 1, 2, 3m) on recovery at acid concentration of 40 g/l and solution flow rate of 0.1 L/m2/min, amount of uranium contained in the ore is directly proportional to the column height. The obtained results indicated that the rate extraction percentage (83.9%) for uranium at 1.0 m column height while its given 81.5% uranium extraction at 2.0 m column height but the lowest uranium extraction was obtained at 3.0 m column height. An inverse relation exists between the height of the heap and the percentage of extraction, confirming the observation of Lizama et al. about the inverse relationship between recovery and heap height [17]. If the height of the heap is lowered in order to increase the percentage recovery, it is necessary to increase the area of the heaped mineral (pad), and a larger volume of solution is required to maintain the same irrigation rate and the same charge capacity. This may be due to the contain significant quantities of fine particles and clay minerals which could contribute to heap leaching difficulties
Application of column optimum conditions on column leaching of uranium: Column leaching studies were performed on representative samples from El-Sela. The leaching process of uraniferous mineralization was conducted with columns being charged with 120 kg mineralized sample. The average uranium content is 0.14% while the particle sizes is less than -10mm and the acid concentration between 20 and 70 g/l and the sprinkling intensity was 0.1 L/m2/min, while the uranium mineralization height on column was 2.0 m. Daily effluent solutions were collected and analyzed for uranium in solution and an extraction profile generated. The results obtained appear in Table 7 below and in Figure 5 as well. From the obtained data of leaching column experiments that has extended for 38 day, it was found that a total leached uranium amount attaining about 136.9 g and about 4080 g acid has been consumed (34 g acid/Kg ore) using the optimum conditions. In the meantime, an overall leaching efficiency of about 81.0% has been obtained from El-Sela sample mineralization, in comparison with the original uranium concentration 1400 ppm.
| Days | Lixiviant | Leaching liquor | Remark | ||||
|---|---|---|---|---|---|---|---|
| Volume L |
H2SO4, g/l |
Volume, ml |
pH | U, mg/l |
U, Extracted, g |
||
| 1 | 10 | 40 | |||||
| 2 | 10 | 40 | 3900 | 5.0 | 1900 | 7.41 | |
| 3 | 10 | 40 | 5100 | 4.5 | 2200 | 11.22 | |
| 4 | 10 | 40 | 5500 | 3.0 | 2300 | 12.56 | |
| 5 | 5 | 40 | 7900 | 2.0 | 2300 | 18.17 | |
| 6 | 8 | 40 | 11400 | 2.0 | 1800 | 20.52 | |
| 7 | 8 | 40 | 3500 | 2.0 | 1600 | 5.60 | |
| 8 | 2.0 | not finish | |||||
| 9 | 8 | 40 | 13000 | 2.0 | 1200 | 15.6 | |
| 10 | 1.7 | Recycle | |||||
| 11 | 8 | 20 | 6300 | 2.0 | 1450 | 9.13 | |
| 12 | 2.0 | not finish | |||||
| 13 | 8 | 20 | 5000 | 2.0 | 1400 | 7.00 | |
| 14 | Recycle | ||||||
| 15 | 8 | 20 | 12650 | 1.5 | 1000 | 12.65 | |
| 16 | 1.5 | Recycle | |||||
| 17 | Recycle | ||||||
| 18 | 8 | 20 | 7200 | 1.5 | 890 | 6.4 | |
| 19 | 1.5 | Recycle | |||||
| 20 | 1.5 | Recycle | |||||
| 21 | 1.5 | Recycle | |||||
| 22 | 8 | 20 | 6300 | 1.0 | 750 | 4.725 | |
| 23 | 1.5 | Recycle | |||||
| 24 | 1.5 | Recycle | |||||
| 25 | 1.5 | Recycle | |||||
| 26 | 8 | 20 | 7800 | 1.5 | 500 | 3.9 | |
| 27 | 1.5 | Recycle | |||||
| 28 | 1.5 | Recycle | |||||
| 29 | 1.5 | Recycle | |||||
| 30 | 8 | 20 | 6500 | 1.5 | 230 | 1.495 | |
| 31 | Recycle | ||||||
| 32 | 1.5 | Recycle | |||||
| 33 | 1.5 | Recycle | |||||
| 34 | 1.5 | Recycle | |||||
| 35 | 1.5 | Recycle | |||||
| 36 | 4 | 0 | 7000 | 1.5 | 50 | 0.35 | Washing |
| 37 | 4 | 0 | 1.8 | Washing | |||
| 38 | 0 | 0 | 8500 | 1.9 | 30 | 0.255 | Finish |
| 133 | 117.5 | 136.88 g | |||||
Table 7: The Results of column leaching of El-sela mineralization.
Uranium recovery from El-sela leach liquor:Acid leaching is a non-selective process, which results in the dissolution of elements other than uranium and the production of large volumes of low-grade solution. Ion exchange technique is an effective method to concentrate and purify uranium from leaching liquor, this exchange provides a means for highly selective recovery.
Uranium adsorption results: During acid leaching, uranium is presented in dilute sulfuric acid solution as the form of uranium sulfate complex anions. Generally, ion exchange resins used in the uranium industry are of the strong base anionic type. This type of resin is made up of a fixed ion group and a mobile ion. Two column resin ion exchange technique was adopted for the recovery of uranium. Anionic complexes of uranium [(UO2(SO4)3]4- were selectively adsorbed by the D263B Chinese resin from leach liquor. The barren liquor was collected separately. The barren solution analyzed: U3O8-~20 mg/l; Fe-13869 mg/l; Mn- 686 mg/l; Pb -3.6 mg/l; Cd–6.1 mg/l; V-28 mg/l; SO42- 15.5 g/l; TDS - 24 g/l and Al- 11473 g/l.
The results of the adsorption experiment for El-Sela pregnant uranium solution assaying 1710 mg/L, 8.5% Fe and pH=1.6 its corresponding adsorption curve is shown in Figure 6. El-Sela solution has regular adsorption curve and D263B resin presents a good suitability of adsorption, and has desirable loading characteristics for the feed solution. So, this resin can be used to recover uranium from El-Sela leaching liquor. The ratio of the breakthrough volume (BV=55) to the saturation volume (SV=80) is an important characteristic, which reflects the efficiency of the resin. The BV/SV is more than 0.5 for ElSela solution. The latter value (0.68) for BV/SV is considered suitable for uranium recovery operation. The obtained adsorption efficiency was 85.5% for El-Sela pregnant solution.
Uranium elution from El-Sela: Sodium chloride has the advantage of low cost and plentiful resource, so sodium chloride (NaCl) is selected as eluent in stripping. Acid is added to the eluting reagent to prevent precipitation of eluted ions by hydrolysis, and to hasten removal of slower eluting ions from the resin. Typical reactions during eluting can describe as follows:
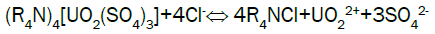
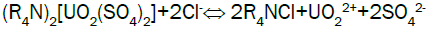
The elution results for El-Sela loaded resin column are shown in Figure 7. From the above-mentioned results, rich-eluate has a higher concentration of uranium. This is due to the higher assay of uranium in the working solution of 1710 ppm. Average uranium concentration of the rich-eluate fractions herein is 20.2 g/L, which is quite suitable for uranium precipitation, while the uranium elution efficiency reached 86.5%. Also, the elution curve has a sharp peak, demonstrating that higher eluate concentration can be obtained by less volume of eluent, and illustrating that this resin has good elution characteristics.
Precipitation
To prepare a uranium sample product from El-Sela ore material, uranium fractions were collected and then precipitated by sodic decomposition. The collected uranium eluted from El-Sela, 10% NaOH was added till pH 6.5-7.5. A bright yellow precipitate (yellow cake) of sodium di-uranate (Na2U2O7) was obtained. After drying, analysis of a sample product resulted in 58%uranium. Finally, a tentative flow sheet is proposed for the treatment of the worked uraniferous ore material (Figure 8). starting with column leaching using 40 g/l H2SO4 and ended with uranium precipitation as sodium di-uranate (Na2U2O7).
It is demonstrated by the laboratory experiment that heap leaching is suited to process El-Sela uranium mineralization, D263B strong base anion resin can effectively concentrate and purify the obtained pregnant solutions to produce quality product, namely yellow cake. Based on the experimental results, relevant processing parameters are acquired and recommended to design El-Sela experimental yellow cake production unit. Experimental processing parameters are summarized in Table 8.
| Items | El-Sela |
|---|---|
| Uranium grade | 1400 |
| Grain size, mm | -10 |
| Leaching acidity, g/l | 20~70 |
| Leaching efficiency, % | 81.5 |
| Leaching period, d | 38 |
| Consumption of H2SO4, Kg/t | 34 |
| Resin type | Anion exchange(D263B) |
| Eluant | 1.0 M NaCl+0.1 M H2SO4 |
| Precipitant | 10%~20% NaOH |
Table 8: Processing parameters of column laboratory study (120 Kg).