e-ISSN: 2319-9849
e-ISSN: 2319-9849
State Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemistry and Chemical Engineering, Nanjing Tech University, Nanjing, Jiangsu, PR China KAUST Catalysis Center, King Abdullah University of Science & Technology, Thuwal, Saudi Arabia
Received date: 11/09/2015; Accepted date: 28/09/2015; Published date: 30/09/2015
Visit for more related articles at Research & Reviews: Journal of Chemistry
Using a corrugated paper as the substrate, a novel compact monolith catalyst assigned as V2O5-WO3/TiO2 for vessel De-NOx application was prepared by wash coating method. The prepared corrugated paper catalyst exhibited comparable NO conversion but lower SO2/SO3 conversion than that of commercial catalyst at low temperature of 200 and 250°C.
In order to meet the requirement of International Maritime Organization (IMO) announced NOx Tier III regulations on vessels, the selective catalytic reduction (SCR) with NH3 at low temperature <250°C has been widely studied over various catalysts [1-2]. Among those catalysts, the V2O5–WO3/TiO2 is the most representative catalyst [3].
For mobile vessel NH3–SCR system, it is obvious that, not only the good reactivity but also compact property like low bulk density is required for the catalyst. However, surprisingly, to our knowledge, most literatures focused only the catalysts reactivity performance, but neglecting the compact issue of the catalysts system. In light of these, a commercial corrugated paper is applied here as the substrate to prepare V2O5–WO3/TiO2 catalyst by wash-coating method. The catalyst performance was evaluated at both 200 and 250°C to investigate its application possibility for the vessel NH3–SCR process.
A commercial corrugated paper (Dongguan Chunhing paper Co., Ltd) was used as a substrate in Figure 1(a). This corrugated paper has a 790 m2/m3 contact area per volume, which is comparable to commercial honeycomb catalyst, whereas the bulk density is only 60% of the commercial honeycomb catalyst value. The composition of this material is SiO2, Al2O3, MgO and CaO. A wash–coating method with the slurry containing commercial WO3-TiO2 and Ammonium vanadium oxide was employed to prepare catalysts in this study. The V2O5 loading was controlled at 3.8 wt. %. The prepared sample was calcined at 450°C for 5 h.
As a control sample, a commercial V2O5–WO3/TiO2 honeycomb catalyst with 3.8 wt. % V2O5 loading shown in Figure 1(b) was provided by Jiangsu Longyuan Catalyst Co., Ltd.
Catalysts CharacterizationThe elemental composition was determined by the AES–ICP in a Thermo–Electron model 3580 instrument. The XRD was recorded on a Bruker D8 Advanced A25 diffractometer. SEM images were taken by the FEI Quanta 200. The XPS analyses were conducted on a MultiLab ESCA 2000 X-ray photoelectron spectrometer with Mg–Kα radiation at 300 W.
Catalysts ReactivityThe catalysts evaluation was performed over a fixed bed reactor. A 57.6 mL prepared corrugated paper catalyst or commercial honeycomb catalyst was loaded into a reactor with O.D.60.5 mm and I.D. 52.9 mm. The catalyst performance is evaluated with both NO conversion and SO2/SO3 conversion. For the De-NOx activity test, a stimulated gas composed of 180 ppm NH3, 180 ppm NO, 7% O2, 10% H2O with N2 balance was applied at SV 28125 h-1. For the SO2/SO3 oxidation test, 500 ppm SO2, 4% O2, 10% H2O with N2 balance gas was applied at SV 7734 h-1.
The rate constant KNO is used here to compare prepared corrugated paper catalyst and commercial honeycomb catalyst [4]:
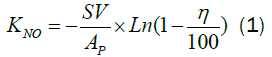
Where, AP is the contact area per volume, η is the NO conversion, SV is the space velocity.
The prepared catalyst in Figure 2(a) showed the similar XRD pattern as that of commercial WO3–TiO2 powder in Figure 2(b), while negligible V2O5 peak could be detected. This implied that the vanadium oxides are well dispersed. The WO3 refraction peak was only shown after calcining the commercial WO3–TiO2 powder at 1100°C to transform the anatase TiO2 to rutile TiO2 in Figure 2(c).
XPS Results in Figure 3 further explained the existence of anatase TiO2 and WO3 over prepared catalyst. The existence of 45% V5+, 45% V4+ and 10% V3+ were detected also. According to Youn et al.[5], the existence of V3+ and V5+ in XPS most likely form the polymerized species, while the V4+ may result in both isolated and polymerized surface vanadium species. Due to the higher liability of lattice oxygen atoms, the polymeric vanadium species were reported to be 10 times more active than the monomeric species at low temperature 200–250°C. The influence of the V state over the De-NOx activity at lower temperature will be discussed in detail in our other study.
Effect of Coating ThicknessThe influence of coated catalyst layer thickness on the De–NOx activity was investigated in Figure 4. At both 200 and 250°C, the De-NOx activity increased significantly with increasing the catalyst layer thickness until 0.2 mm. Continuing increase the catalyst layer thickness from 0.2 to 0.4 mm, negligible improvement of De-NOx activity was evidenced. This suggested that the De- NOx reaction was confined into a narrow surface layer. Yang et al. reported that over a honeycomb monolith support wash coated SCR catalyst, the activity increased with increasing the coating thickness and reached at 0.11 mm thickness as comparable as that of the 100% active components-molded catalyst [6].
Figure 5 showed the performance comparison Results in terms of KNO and SO2/SO3 conversion over corrugated paper coated catalyst (catalysts layer thickness 0.2 × 2 mm) and commercial extruded honeycomb catalyst (catalysts layer thickness 0.8 mm). The prepared corrugated paper catalyst exhibited comparable NO conversion but lower SO2/SO3 conversion than that of commercial catalyst. It is known that, the reduction of NO depends mainly on the external geometric surface area and therefore is little influenced by the change of the catalyst layer thickness. But, the conversion of SO2 to SO3 occurs in the whole catalytic volume and thus can be reduced by decreasing the thickness of the active phase layer [7]. However, we must notice that the V2O5- WO3/TiO2 catalyst is weak in the presence of SO2 in the temperature range of 200 and 250°C. And further research to improve its resistance against SO2 is under progress by modifying the catalysts with Mo.
The compact issue of the De–NOx catalyst for vessel is always neglected by many researchers, a novel catalyst V2O5–WO3/ TiO2 based on the corrugated paper support with low bulk density but good reactivity was proposed in this study. With increasing the coated catalyst layer until 0.2 mm, the De-NOx activity increases significantly. Comparing with a commercial extruded honeycomb catalyst with 0.8 mm catalyst layer thickness, the prepared catalyst in this study with 0.2 × 2 mm catalyst layer thickness showed better performance in terms of KNO and SO2/SO3 conversion.