e-ISSN: 2319-9849
e-ISSN: 2319-9849
Designer Energy Ltd, 2 Bergman Str., Rehovot, Israel
Received date: 15/04/2014; Revised date: 26/04/2014; Accepted date: 13/05/2014
Visit for more related articles at Research & Reviews: Journal of Chemistry
The structure of initial and ground samples of chitin and chitosan have been studied using a wide-angle X-ray scattering (WAXS) and sorption of water vapor. To determine the crystallinity degree, an improved WAXS method was used based on the calculation of the relationship between integrated intensities of X-ray diffraction from crystalline and amorphous domains. The calculations revealed that the actual degree of crystallinity of initial chitin was 0.72, and of initial chitosan sample was 0.57. After ball-grinding in a short time, the decrease of the crystallinity degree was observed, whereas the prolonged grinding resulted in complete amorphization of the samples. Sorption of water vapor for chitin, containing hydrophobic acetyl groups was lower than for chitosan, containing hydrophilic groups. The sorption ability of the samples was inversely proportional to their degree of crystallinity. Reduction of the crystallinity degree promoted to the increase in sorption ability of the samples. This evidences that sorption mechanism of water vapor is absorption of the water molecules into amorphous domains of the hydrophilic polymers. The crystallinity degree calculated from results of water vapors sorption was close to the crystallinity degree of the samples obtained by the WAXS method.
Chitin, Chitosan, Crystallinity degree, Sorption ability, Correlation
Chitin is the most abundant cellulose derivative consisting of 1,4-β-N-acetyl-2-aminodeoxyglucose units [1,2]. In the nature, chitin is found in exoskeletons of arthropods and insects, shells of mollusks, pens and beaks of cephalopods, cell walls of some fungi, etc. Resources of this polymer are estimated in 100 billion tons [3]. Commercially, chitin can be isolated from shells of crabs, shrimps and lobsters by acidic removal of calcium carbonate followed by alkaline extraction of proteins and bleaching [2]. Chitin is the main feedstock for the production of such cellulose derivative as 2-aminodeoxycellulose or chitosan by alkaline deacetylation process. Natural chitosan occurs in cell walls of some fungi, e.g. Mucoraceae [3].
Chitin and in particular chitosan are of commercial interest because of unique properties such as low toxicity, antibacterial activity, biocompatibility, biodegradability and sorption ability [1,2,4-7]. Currently, these polymers have a wide range of applications, including medicine, cosmetics, biotechnology, biocomposites, papermaking, agriculture, water purification, etc. Therefore, samples of chitin and chitosan require a detailed structural characterization. The various methods are used for determination of molecular weight, acetyl- and amino groups, humidity, content of residual proteins and minerals, etc [2,6].
Study of the supramolecular structure of chitin and chitosan showed that these cellulose derivatives are linear semicrystalline polymers [8,9]. The linear macromolecules joined by hydrogen bonds form the supramolecular structure of these polymers that consists of nanofibrillar bundles called microfibrils. Each microfibril is built of ordered crystallites and low-ordered non-crystalline (amorphous) domains statistically alternated along the fibril [9]. X-ray studies showed that crystallites of chitin can be in α- and β-polymorph forms [10-12]. The α-form is the most abundant; this form is present in chitin samples isolated from crabs, shrimps, krill, lobsters, insects, as well as fungal and yeast cell walls. The rare β-polymorph is found in chitin samples isolated from squid pens and tubeworms and some others sources.
Crystalline unit cell of α-chitin is orthorhombic with space group P212121, while β-chitin has monoclinic crystalline unit cell with group P21 [10-12]. The β-form of chitin is instable and can undergo an intra-crystalline swelling [13]. Moreover, after some treatments the β-form of chitin is transformed irreversibly into more stable α-polymorph [14]. X-ray diffractograms of the chitin samples show two sharp diffraction peaks at 2θ of about 9-9.5° from (020) planes and 19-19.5° from (110) planes of crystalline unit cells and some weak peaks. Crystalline unit cell of chitosan is also orthorhombic with space group P212121, while X-ray diffractogram has two diffraction peaks at 2θ of about 10-10.5°from (020) planes and 20-20.5° from (110) planes [15]. Thus, as a result of deacetylation of chitosan the crystalline peaks are moved to a higher 2θ angle [16].
However, such structural characteristic of chitin and chitosan as the content of the crystalline phase or the degree of crystallinity has been studied insufficiently. Main reason is that most studies are limited by evaluation of so called index of crystallinity (CrI), based on calculations of the ratio of peaks heights: CrI = (Io – Iam)/Io, where Io is height of the crystalline peak and Iam is height of amorphous scattering [16,17]. Index of crystallinity shows the comparative content of crystalline fraction in several samples. It may indicate which of the samples has greater crystallinity and which less crystallinity, but it does not disclose the actual degree of crystallinity, i.e. weight part of the crystalline fraction in the polymer. CrI of the chitin and chitosan samples was calculated by different ways using heights of crystalline X-ray diffraction peaks from (110) or (020) planes and heights of amorphous scattering at 2θ of 12°, 12.6° or 16° with or without subtraction of the parasitic background scattering [16-21]. The other indexes of crystallinity were proposed based on the dividing the total area of X-ray diffractogram by the crystalline and background areas [22]. Study of crystallinity index of chitin samples showed that depending on the calculation method for the same sample different values of CrI from 0.57 to 0.93 can be obtained [23,24]. Crystallinity index of chitosan samples calculated by different methods can also vary within a wide range from 0.4 to 0.8 [20,25-27]. Thus, results about the crystallinity of chitin and chitosan samples based on estimation of CrI are doubtful.
To determine the actual degree of crystallinity, the quantitative X-ray phase analysis should be performed, which requires compliance with certain conditions, and namely [28]:
- The sample must be in a non-textured powder form;
- The parasitic X-ray background should be subtracted;
- The Experimental diffractogram should be corrected;
- The scattering areas related to crystalline and non-crystalline domains should be separated from the corrected diffractogram;
- The integrated intensity (area) of crystalline and non-crystalline scatterings should be used to calculate the degree of crystallinity.
Indirect method for estimation of the crystallinity is a measurement of hydrophility of the polymer samples. Since sorption of water vapor occurs in non-crystalline (amorphous) domains of the hydrophilic polymers, less crystalline sample will absorb more water vapor, and vice verse. Investigations of water vapor sorption by chitosan samples revealed that the sorption ability decreases with increasing of deacetylation degree (DD) of the samples [29]. This behavior was explained by the influence of crystallinity of the samples on the sorption ability.
Thus, despite the abundance of publications, data about the crystallinity of chitin and chitosan samples is uncertain. This complicates the understanding of the real structural organization of these polymers and its change after various treatments. Therefore, main aim of this paper was determination of actual crystallinity degree of chitin and chitosan samples and establishing a quantitative relationship between the crystallinity degree of the samples and their sorption ability.
Purified powders of chitin isolated from crab shells and chitosan (MW=400 kDa; DD=85%) were acquired from Sigma-Aldrich. To reduce the crystallinity, the initial samples were ground in laboratory ball-mill using ceramic balls for 5 and 24 hours at 250 rpm.
Wide-angle X-ray scattering (WAXS)The dry powders of the equal mass (250 mg) are pressed into tablets (diameter 15 mm, thickness 1.5 mm) that were used for WAXS-experiments. Diffractograms of the samples were recorded in the 2Θ angle range from 5 to 50° using a Rigaku-Ultima Plus diffractometer (CuKα –radiation, λ=0.15418 nm). After recording of the diffractograms, the parasitic background (bg) caused by air, apparatus, Compton scattering, thermal agitation of atoms and molecules and distortions of crystalline lattice was subtracted. To correct of the diffractogram, the X-ray intensities were divided into the correction coefficient, K(θ), that includes the Lorentz-polarization factor (LP) and initial intensity of the X-ray beam (J): K(θ) = JLP [28]. Further, the total integrated intensity (total area) of the corrected diffractogram was separated into areas of crystalline (Cr) and amorphous (Am) scatterings (Fig. 1, 2). For this purpose, a similar transfer of the profile of X-ray scattering of the amorphous sample was carried out (Fig. 3). Finally, the actual degree of crystallinity (X) of the sample was calculated, as follows:
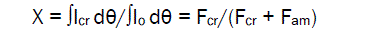 (1)
(1)
where Io is total intensity of the corrected diffractogram after subtraction of the parasitic background (bg); Icr is intensity of the crystalline scattering; Fcr is area of the crystalline scattering; Fam is area of the amorphous scattering.
For comparison, the index of crystallinity (CrI) also was estimated after prior removal of the background:
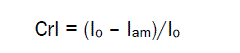 (2)
(2)
where Io is height of the (110)-peak and Iam is height of amorphous scattering at 2θ = 16° [20].
Water vapor sorptionThe sorption experiments were carried out at 25°C using a vacuum Mac-Ben apparatus having helical spring quartz scales. Prior to starting the experiments the samples were dried at 105°C up to constant weight and additionally degassed in the sorption device.
X-ray diffractogram of initial chitin sample was typical for α-polymorph having two expressed peaks at 2θ of 9.2° and 19.0° (Fig. 1, 4). These peaks appear as a result of X-ray diffraction from (020) and (110) planes of the crystalline lattice with inter-plane distances of 0.96 nm and 0.47 nm, correspondingly. Initial chitosan sample gave the diffractogram having a weak peak at 2θ of 10° and more intense peak at 2θ of 20° that caused by diffraction from (020) and (110) planes of the crystalline lattice with inter-plane distances of 0.88 nm and 0.45 nm, correspondingly (Fig. 2, 5).
After ball-grinding of the initial samples in a relative short time (for 5 h), the intensity of the diffraction peaks decreased that indicated on partial decrystallization of the samples (Fig. 4, 5). Prolonged grinding (for 24 h) resulted in complete decrystallization of the samples and appearance wide diffractograms having one maximum at 2θ ≈ 20o typical for amorphous phase state of the polymers (Fig. 3).
Calculations carried out by the equation (1) based on the improved WAXS-method revealed that actual degree of crystallinity of initial chitin was 0.72, and of initial chitosan sample was 0.57 (Table 1). Short-time grinding (for 5 h) caused the decrease in the crystallinity degree of the samples to 0.40-0.45, whereas prolonged grinding (for 24 h) resulted in the complete amorphization of the samples. However, calculations of crystallinity index gave significantly higher values that do not correspond to actual content of the crystalline phase in the samples. Even for the amorphous samples the index of crystallinity was above zero, 0.42 to 0.46, which is clearly erroneous.
Sorption of vapors is usually described through isotherms that indicate the relative amount of sorbate in the sorbent (A, g/g) as a function of the relative vapor pressure (φ = p/po) at a constant temperature (e.g. 25°C). The experiments showed that isotherms of water vapor sorption by chitin and chitosan samples had a sigmoidal shape similar to sorption isotherms of this sorbate by cellulose (Fig. 6, 7). The sorption ability of more crystalline initial samples was less than of partially or completely amorphized samples (Fig. 8, 9). Thus increasing of the crystallinity degree leads to reducing in sorption ability of the samples.
Comparison of sorption isotherms for amorphous samples showed that the sorption ability of chitin containing hydrophobic acetyl groups was lower than chitosan containing only hydrophilic hydroxyl and amine groups.
In order to linearize the sigmoidal isotherms, the following thermodynamic equation can be applied [30]:
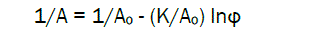 (3)
(3)
where Ao is maximal sorption value at φ = 1; K is coefficient.
Example of linearized isotherms of the water vapor sorption by amorphized samples is shown on Fig. 10.
From the linearized isotherms the sorption parameters Ao and K were found. This permits to calculate the sigmoidal isotherms using the following equation:
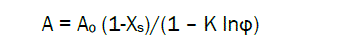 (4)
(4)
where for chitin samples Ao = 0.4 g/g; K=2.7, while for chitosan samples with DD=85% Ao = 0.5 g/g; K=2.7; Xs is degree of crystallinity.
By means of eq. (4) degree of crystallinity (XS) of the sample can be determined from sorption experiments. For this purpose it is convenient to use the sorption value A0.5 (g/g) at the relative pressure of vapors φ = 0.5. Then:
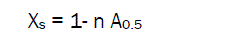 (5)
(5)
where for chitin samples coefficient n= 7.15 and for chitosan samples with DD=85% n= 5.65.
The crystallinity degree calculated from results of water vapor sorption correlates with this structural characteristic obtained by the WAXS method (Table 2).
As follows from the investigations, the sorption ability of amorphized samples is higher than of crystalline samples. Thus, reduction in the crystallinity degree and rise in the content of amorphous domains contributed to increase of sorption ability of the sample. This evidences that sorption mechanism of water vapor is absorption of the water molecules into amorphous domains of the hydrophilic polymers.
Crystallinity is an important structural characteristic influencing the various properties of polymers. This characteristic permits also to understand the supramolecular structure of the polymers. Unfortunately, the crystallinity of chitin and chitosan samples has been measured by the insufficiently correct method using the ratio of the height of crystalline diffraction peak and the height of amorphous scattering at 2θ of 12°, 12.6° or 16°. However, the experiments showed that the maximum of amorphous X-ray scattering of the samples is observed at 2θ ≈ 20°, but not in the 2θ range of 12-16°. Besides, evaluation of the crystallinity from the ratio of peaks heights is not justified theoretically because the peak height is not proportional to the content of crystalline or amorphous phase. The law of phase proportionality is observed only, when integral intensities (areas) of the X-diffraction are used [28]. In this study to determine the crystallinity an improved method was used based on the calculation of the relationship between integrated intensities of X-ray diffraction from crystalline and amorphous phases. The calculations revealed that actual degree of crystallinity of initial chitin was 0.72, and of initial chitosan sample was 0.57. After ball-grinding in a short time, the decrease of the crystallinity degree of the samples was observed, whereas the prolonged grinding resulted in the complete amorphization of the samples. In contrast to degree of crystallinity, the index of crystallinity gave significantly higher values that do not correspond to actual content of the crystalline phase in the samples.
Isotherms sorption of the water vapor by chitin and chitosan samples had a sigmoidal shape, which can be described by a thermodynamic equation: A = Ao (1-Xs)/(1 – K lnφ); where φ is relative vapor pressure; Ao is maximal sorption value at φ = 1; Xs is degree of crystallinity; K is coefficient. Experiments showed that the sorption ability of chitin containing hydrophobic acetyl groups was lower than chitosan containing only hydrophilic hydroxyl and amine groups.
As follows from the investigations, the sorption ability of the samples was inversely proportional to their degree of crystallinity. Decrease of the crystallinity degree and rise of the content of amorphous domains promoted to increase of sorption ability of the samples. This evidences that sorption mechanism of water vapor is absorption of the water molecules into amorphous domains of the hydrophilic polymers.
The crystallinity degree calculated from results of water vapors sorption was close to the crystallinity degree of the samples obtained by the WAXS method.