ISSN: 2320-2459
ISSN: 2320-2459
1Department of Physics, Banasthali University, Rajasthan, India.
2Department of Physics, Malaviya National Institute of Technology Jaipur, Rajasthan, India.
Received date: 13/12/2013; Revised date: 02/01/2014; Accepted date: 11/01/2014
Visit for more related articles at Research & Reviews: Journal of Pure and Applied Physics
The presented paper deals with crystallization behavior of Polyethylene Terephthalate (PET)-ZnO/TiO2 nanocomposites. ZnO & TiO2 nanoparticles have been synthesized by chemical method and the nanocomposites with PET have been prepared by solution casting method. The XRD analysis of ZnO and TiO2 nanoparticles illustrate the average particle size as 18.93 nm and 19.31 nm, respectively. The DSC thermograms of PET- ZnO/TiO2 nanocomposites explain the crystallization behavior when the samples have been subjected to cooling after melting. The increment in cooling rate causes to lower the crystallization peak temperature. By applying Kissinger model, the crystallization activation energy could be determined. ZnO nanoparticles have been proved to be more efficient than TiO2 nanoparticles for heterogeneous nucleation in PET matrix
Polymer nanocomposites, XRD, crystallization, activation energy.
Polymers play an essential and ubiquitous role in everyday life due to the extraordinary range of properties of polymeric materials. In the vast field of nanotechnology; polymer matrix based nanocomposites have become a prominent area of current research and development [1,2]. Among all the polymers, Polyethylene Terephthalate (PET) has drawn considerable interest as a packaging material for soft drinks, juices, alcoholic drinks, water, edible oils, household cleaners and other food and non-food applications [3]. Being semicrystalline thermoplastic polyester [1], the thermal stability and crystallization property can be enhanced in its nanocomposites with metal oxides. The degree of crystallinity affects the extent of the intermolecular secondary bonding of polymer [4] and as a result, increment in degree of crystallinity improves the tensile modulus and its strength. In addition, the material tends to become more brittle. Metal oxides work as nucleating agent in the process of heterogeneous nucleation and growth which is fundamental fact of non isothermal crystallization.
Titanium dioxide (TiO2) and Zinc Oxide (ZnO) are commonly used inorganic fillers for organic polymer matrix. These are wide band gap (Eg (TiO2) =3.2eV, Eg (ZnO) =3.37 eV) semiconductor materials [5,6]. The use of TiO2 is advantageous over capping agents in the chemical reduction process of metal ions, as it is free from the blocking of active sites by organic capping agents [7]. In addition, ZnO and TiO2 nanofillers causes to lowering the crystallization activation energy of PET that is required to initiate crystallization process under non isothermal crystallization kinetics.
In this paper, we focus on the synthesis of PET-ZnO/TiO2 nanocomposites by solution casting method and the crystallization activation energy of these nanocomposites with the help of Diffrantial Scanning Calorimeter (DSC) thermograms.
Synthesis of ZnO nanoparticles
ZnO nanoparticles were synthesized using a chemical route [8]. Firstly, 0.2 M zinc acetate ((CH3COO)2Zn.2H2O) was dissolved in 20 ml Di-methylene sulfoxide (DMSO), and 1.2 M Potassium hydroxide (KOH) was dissolved in 10 ml ethanol. After stirring both solutions for 30 min separately, the zinc acetate solution was mixed in KOH solution drop wise. Then 1.2 ml thioglycerol was added in mixed solution and stirred for 1 hr. The solution becomes milky, that was washed with methanol and distilled water. This washed milky solution was centrifuged to get white precipitate that was dried at room temperature.
Synthesis of TiO2 nanoparticles
Synthesis of TiO2 nanoparticles was carried out using a typical chemical precipitation method [5]. TiCl3 solution was mixed with NH4OH aqueous solution in 1:6 volume ratios. The resulting solution has been stirred for 48 h at room temperature. By centrifuging solution, white precipitate was further washed in iso-propyl alcohol and dried at room temperature. This procedure produced white colored dry nanopowder.
Preparation of PET-ZnO/TiO2 nanocomposites
PET- ZnO/TiO2 nanocomposites have been prepared by solution casting method [9]. In a typical procedure for preparation of nanocomposites having 0.5 wt% TiO2 nanoparticles, PET has been dissolved in Di-chloro methane (DCM) with Tri-fluoro acitic Acid (TFA). Accordingly, 0.5% ZnO nanoparticles have been added in polymer matrix and stirred for 2 h at room temperature. This solution has been poured to petri dish at room temperature. Using similar procedure, nanocomposites for 1 and 1.5 wt% TiO2 nanoparticles have been prepared. The same method was followed for PET-TiO2 nanocomposites with 0.5, 1, and 1.5 wt% TiO2 nanoparticles.
Non-isothermal crystallization kinetics of TiO2 and ZnO nanoparticles filled composites of PET has been studied using NETZSCH DSC 204 F1 Phoenix. The samples were heated up to 300 °C with different heating rates (5, 10, 15 and 20K/min) under nitrogen atmosphere of 40-50 ml/min, and held at the maximum temperature for 5 min to remove its previous thermal history. The crystallization kinetics has been investigated by cooling these samples from 300 to 30 °C with the same rate.
The XRD patterns of TiO2 and ZnO nanoparticles were obtained using X-ray diffractometer Bruker D8 Advance with Cu (Kα) radiation in the range of 20°-70° (λ=0.154nm) to confirm the crystallite size. Figure 1 shows the XRD graph for ZnO and TiO2 nanoparticles, respectively. The average particle size of ZnO and TiO2 nanoparticles have been determined using Debye-Scherrer’s formula [10] and come out to be 18.93 nm and 19.31 nm, respectively.
The crystallization curves at various cooling rates for neat PET, PET+1%TiO2 and PET+1%ZnO have been illustrated in figure 2(a), 2(b) and 2(c), respectively as a representative case. It is clearly observed that for all samples, as cooling rate is increased, the crystallization onset temperature (Ts), peak temperature (Tc) and end temperature (Te), shift to lower temperature side. The values of melting temperature (Tm) and crystallization peak temperature (Tc) have been listed in table 1.
A particular amount of energy is required to initiate this crystallization phenomenon that is said to be crystallization activation energy. For evaluation of activation energy (E) of non-isothermal crystallization kinetics at various cooling rates, the model proposed by Kissinger [11] is commonly used;
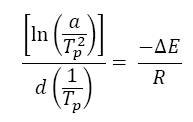 (1)
(1)
Where, R is the universal gas constant, a is the cooling rate and Tp is crystallization temperature.
Figure 3 shows the plot between ln(a/Tp2) and 1/Tp for PET-TiO2 and PET-ZnO nanocomposites and, accordingly the crystallization activation energy could be determined by slope of the plots as listed in table 2. The results verify that ZnO and TiO2 nanoparticles are working as favorable nucleating agent under non isothermal crystallization. The comparative study of crystallization activation energy reveals TiO2 and ZnO nanoparticles cause to reduce activation energy. But ZnO nanoparticles are more active than TiO2 nanoparticles in nucleation and growth process for PET matrix. The less energy is required for crystallization of PET-ZnO nanocomposites. As the ZnO and TiO2 nanofiller contents in PET matrix are increased, the crystallization activation energy decreases.
The synthesis of TiO2 and ZnO nanoparticles have been carried out using simple chemical method and the average particle diameter has been calculated by XRD patterns with the help of Debye-Scherrer’s formula. The nanocomposites of PET with both TiO2 and ZnO nanoparticles have been prepared via solution casting method. The DSC thermograms show crystallization behavior of nanocomposites during cooling cycle. With the increased cooling rate, the crystallization temperature (Tp), Ts and Te shift to lower temperature side. To determine the crystallization activation energy of PET-TiO2/ZnO nanocomposites, Kissinger model has been applied. Results show that ZnO nanoparticles are more favorable nucleating agent than that of TiO2 nanoparticles in PET matrix under non isothermal crystallization.
Authors are thankful to DST, Govt. of India. DST has granted sophisticated research facilities to Banasthali Vidyapith under its CURIE scheme. One of author (KA) is thankful to DST-New Delhi for funding under INSPIRE faculty award.