e-ISSN:2320-1215 p-ISSN: 2322-0112
e-ISSN:2320-1215 p-ISSN: 2322-0112
Department of Pharmaceutics, Bapatla College of Pharmacy, Bapatla, Andhra Pradesh, India.
Received: 12/06/2013; Revised: 23/06/2013; Accepted: 02/07/2013
Visit for more related articles at Research & Reviews in Pharmacy and Pharmaceutical Sciences
In this new era of novel drug delivery systems, formulation research is oriented towards increasing the efficacy of existing drug molecule through these novel concepts of drug delivery. Losartan potassium is widely used as an antihypertensive drug, which is a potent drug candidate for developing in to Orodispersible tablets (ODTs). It has low bioavailability due to first pass metabolism. Hence the main objective of the study was to formulate fast dissolving tablets of Losartan potassium to achieve a better dissolution rate and further improving the bioavailability of the drug. Orodispersible tablets of Losartan potassium were formulated by using different concentration of superdisintegrants like sodium starch Glycolate, Crospovidone individually, physically mixed and co-processed and all the batches were prepared by direct compression using standard round faced punch on a sixteen station rotary (Cadmach, Ahmadabad) tablet punching machine. Disintegration time and drug release were taken as the basis to optimize the orodispersible tablet. All the tablets were Prepared & evaluated for various parameters like hardness, weight variation, friability, thickness, disintegration time wetting time and dissolution time. Among all the formulations containing 5% w/w of co-processed superdisintegrant (1:1 mixture of CP and SSG) was found to be shown faster and high drug dissolution.
Losartan potassium, Orodispersible tablet, Co-processed excipients.
Losartan potassium is an antihypertensive drug belongs to the category of Angiotensin II receptor antagonist. Losartan potassium is widely used as an antihypertensive drug, which is a potent drug candidate for developing in to Oro dispersible Tablets (ODT’s). It has low bioavailability due to first pass metabolism2. It has a half-life of 1.5-2h. So Oro dispersible tablet formulation avoids the first pass metabolism.
Orodispersible tablets are those solid dosage forms when put on the tongue, disintegrate or dissolve instantaneously, releasing the drug within a few seconds without the need of water [1]. These dosage forms provide fast onset of action, patient compliance, and good chemical stability. Orodispersible tablets are also known as “Mouth dissolving tablets”, “Orally disintegrating tablets”,” Melt- in- mouth”,” Fast dissolving drug delivery”, ” Rapimelts tablets”, “Porous tablets”, Quick dissolving tablets” etc. Orodispersible tablets allows high drug loading and no chewing is needed. In conventional dosage from there is delay in disintegration and therefore dissolution, while ODTs rapidly disintegrates in oral cavity and thus dissolution is fast. Due to disintegration of ODTs in mouth absorption starts at mouth then pharynx and oesophagus. Various approaches to formulate ODTs are lyophilisation, moulding, spray drying, sublimation, direct compression and mass extrusion.
Most of formulations (>75%) contains excipient concentration more than the active drug which increases the bulk of the formulation to an extent of inconvenience for swallowing a tablet. So the single component excipients came into existence but the in recent years drug formulation scientists have recognized that single component excipients do not always provide the requisite performance to allow certain active pharmaceutical ingredients to be formulated or manufactured adequately. In order to overcome this problem there is a need to develop excipients with multiple characteristics built into them such as better flow, low/no moisture sensitivity, superior compressibility and rapid disintegration ability. One of the interesting options for such excipients development is co-processing. Co-processing is based on the novel concept of two or more excipients interacting at the sub particle level, the objective of which is to provide a synergy of functionality improvement as well as masking the undesirable properties of individual, without interacting at chemical level.
In the present study, an attempt was made to develop Oro dispersible tablets of Losartan potassium and investigate the effect of different types of superdisintegrants on the release profile of the drug in the tablets and to improve patient compliance.
Losartan potassium, Sodium starch Glycolate, Crospovidone are obtained from Natco Pharma Ltd, Hyderabad as a gift sample. Iso propyl alcohol, Mannitol, Micro crystalline cellulose are of pharmaceutical grade. Magnesium stearate, talc are purchased from S.D. Fine chem., Mumbai. Gum karaya and banana powder obtained from local market.
Physical mixtures were prepared in ratios from 1:1, 1:2, 1:3 with the help of spatula in mortar.
Blends of Crospovidone and sodium starch glycolate having total weight of 10g were prepared in ratios from 1:1, 1:2, and 1:3 and was added to 60 ml of Iso propyl alcohol. The contents of the beaker (250 ml capacity) were stirred on a magnetic stirrer. The temperature was maintained between 65ºC and 70ºC, and stirring was continued till most of Iso propyl alcohol evaporated. The wet coherent mass was granulated through # 60mesh sieve.
Blend was weighed and transferred to a measuring cylinder. Then bulk volume was noted. Bulk density was calculated by using the following formula.
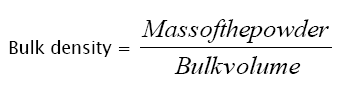
Blend was weighed, transferred to a measuring cylinder and subjected to 100 tapings. Then volume was noted as tapped volume. Tapped density was measured by using the following formula
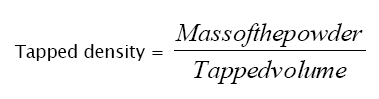
Carr’s index was calculated by using the following formula
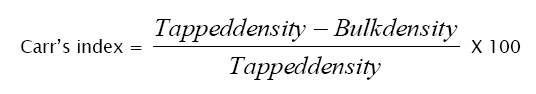
Hausner’s ratio: Hausner’s ratio was calculated by using the following formula
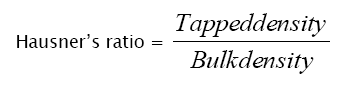
Required quantity of blend was taken and poured into a hollow cylinder which was placed on a graph sheet. Then the cylinder was slowly lifted. Then height and diameter of the heap formed were noted down. The angle of repose (θ) was calculated by the formula.
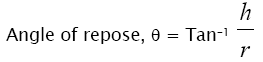
Tablets were made from blends by direct compression method. All the ingredients (shown in Table-1) were passed through mesh no.80. All the ingredients were co ground in a pestle with motor. The resulting blend was lubricated with magnesium stearate and compressed into tablets using the Cadmach single punch (round shaped, 7mm thick) machine.
Weight variation test was done by weighing 20 tablets individually, calculating the average weight and comparing the individual tablet weight to the average weight.
Twenty tablets were powdered, and 25 mg equivalent weight of Losartan Potassium in tablet powder was accurately weighed and transferred into a 100 ml volumetric flask. Initially, 5 ml methanol was added and shaken for 10 min. Then, the volume was made up to 100 ml with 6.8 phosphate buffer solution. The solution in the volumetric flask was filtered, diluted suitably and analyzed spectrophotometrically at 235 nm.
The disintegration time was determined in distilled water at 37±0.50 C using disintegration test apparatus USP ED-2L (Electro lab, Mumbai).
Roche friabilator was used to determine the friability. Pre weighed tablets were placed in friabilator and rotated at a speed of 25 rpm for 4 minutes or up to 100 revolutions. The tablets are dropped from a distance of 6 inches in each revolution. The tablets were then reweighed after removal of fines and the percentage of weight loss was calculated.
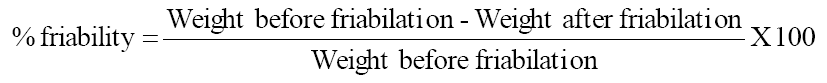
Hardness of the tablet was determined using the Monsanto hardness tester. The lower plunger was placed in contact with the tablet and a zero reading was adjusted. The plunger was then forced against a spring by tuning threaded bolts until the tablet fractured. Then the final reading at the point of fracture was recorded. The hardness was computed by deducting the initial pressure from the final pressure.
Five circular tissue papers of 10 cm diameter are placed in a Petri dish with a 10 cm diameter. 10 mL of water-containing amaranth (a water soluble dye) is added to Petri dish. A tablet is carefully placed on the surface of the tissue paper. The time required for water to reach upper surface of the tablet is noted as a wetting time.
Tablet was added to 10 ml of phosphate buffer solution of pH 6.8 (pH of saliva) at 37± 0.5°C. Time required for complete dispersion of tablet was measured.
This test was performed by placing two tablets in 100 ml of water and stirring it gently, until the tablets get completely disintegrated. Then the dispersion is passed through a sieve screen with a nominal mesh aperture of 710 μm.
Dissolution studies for Losartan Potassium orodispersible tablets were performed in pH 6.8 phosphate buffer using USP dissolution test apparatus (Electrolab, Mumbai, India) with a paddle stirrer. The paddles were allowed to rotate at 100 rpm. The dissolution medium was maintained at a temperature of 37+0.5OC and samples were withdrawn at an interval of 5 min the volume of the withdrawn samples were replaced by fresh dissolution medium in order to maintain the sink conditions. The withdrawn samples were filtered and absorbance was measured at 235nm using UV-visible spectrophotometer.
The drug release data were plotted and tested with zero order (cumulative % drug released Vs time), First order (Log % remained Vs time). The in-vitro dissolution kinetic parameters like dissolution rate constant (K), correlation coefficient(r), the time (T50) for 50 % of drug released and dissolution efficiency (D.E) were calculated. From the slopes of linear plots, the dissolution rates were calculated.
Micromeritic properties of the blends were studied and results were shown in Table- 2. All the blends exhibited good flow properties and are found to be suitable for direct compression.
To study the influence of superdisintegrants on the performance of Losartan potassium, a set of two formulations (F1 and F2) were prepared using two superdisintegrants viz, Sodium Starch Glycolate (5%) and Crospovidone (5%) respectively. The formulated tablets were subjected to various quality control tests and the results were shown in Table-3. All the tablets complied with the pharmacopoeial standards. The dissolution data was presented in Table-4 and Figure-1. The In-vitro dissolution kinetics was presented in Table-5. The dissolution rate followed first-order kinetics (Figure-2) as the graphs drawn between log % drug unreleased Vs time were found to be linear. The dissolution rate of Losartan potassium was found to be effected by nature of the superdisintegrant used in the preparation of tablets. Based on the dissolution rate, efficiency of superdisintegrants is Sodium Starch Glycolate < Crospovidone. The formulation prepared with Crospovidone was offered relatively rapid release of Losartan potassium when compared with Sodium Starch Glycolate.
Co-processed superdisintegrants were prepared by solvent evaporation using Crospovidone and Sodium Starch Glycolate in different ratios (1:1, 1:2, and 1:3). The co-processed superdisintegrants were evaluated for their flow and compression properties in comparison with physical mixture of superdisintegrants. The angle of repose of co-processed superdisintegrants was found to be (25.1-25.7) which indicates excellent flow in comparison to Physical mixture of superdisintegrants (26.1-26.7) due to granule formation. Carr’s index was in the range of 13.27 to 14.88 % and Hausner’s ratio was in the range of 1.15 to 1.17.
The data obtained from post-compression parameters such as hardness, friability, thickness, drug content, wetting time, and in-vitro disintegration time are shown in (Table-2). Hardness of all formulations was ranges from 3.6 to 3.8 kg/cm2. Friability is less than 1%, which indicated that tablets had a good mechanical strength. Drug content was found to be in the range of 98.43 to 99.47 %, which is found to be within the acceptable limits. The wetting time is an important criteria for understanding the capacity of swell to disintegrate in presence of little amount of water were found to be 82 to 99 sec. From both pre and post formulations parameters, a comparative study was performed in between tablets formed by co-processed mixtures and physical mixtures of superdisintegrants. Three formulations (F3, F4, and F5) were made by physical mixture of Crospovidone and Sodium Starch Glycolate (1:1, 1:2, 1:3). Based on the dissolution rate, the order of drug release from the three formulations were F3> F4> F5. The dissolution data was presented in Table-5 and Figure-4. The In-vitro dissolution kinetics was presented in Table-6. Another three formulations (F6, F7, and F8) were made with co processed Crospovidone and Sodium Starch Glycolate (1:1, 1:2, 1:3). The order of drug release for these three formulations was F6> F7> F8. The dissolution data was presented in Table-3 and Figure-6. The In-vitro dissolution kinetics was presented in Table-4. The dissolution rate followed first-order kinetics (Figure-7) as the graphs drawn between log % drug unreleased Vs time were found to be linear. This data reveals, dissolution rate of Losartan potassium influenced by ratios of individual superdisintegrants employed in combination of superdisintegrants in both physical mixing and co processing (1:1, 1:2, 1:3) and type of combination of superdisintegrants (physical mixing Vs co-processing) employed. It was found that the tablets prepared by co-processed mixtures gave better results as compared to those prepared by physical mixtures. Among all the formulations the formulation-F6 containing 5% w/w of co-processed superdisintegrants (1:1 mixture of CP and SSG) was found to be best releasing and has shown an in-vitro dispersion time of 151sec.
All the blends exhibited good flow properties and suited for direct compression. The formulation prepared with 5%w/w of Crospovidone was offered relatively rapid release of Losartan potassium when compared with 5%w/w of Sodium Starch Glycolate. The order of drug dissolved from the three formulations made with physical mixture of Crospovidone and Sodium Starch Glycol ate (1:1, 1:2, 1:3) is
F3 (1:1)> F4 (1:2)> F5 (1:3).
The order of drug dissolved from the three formulations made with co processed mixture of Crospovidone and Sodium Starch Glycol ate (1:1, 1:2, 1:3) is
F6 (1:1)> F7 (1:2)> F8(1:3).
Among all the formulation- F6 containing 5% w/w of co-processed superdisintegrant (1:1 mixture of CP and SSG) was found to be shown faster and high drug dissolution. Dissolution rate of Losartan potassium influenced by ratios of individual superdisintegrants employed in combination of superdisintegrants in both physical mixing and co processing (1:1, 1:2, 1:3) and type of combination of superdisintegrants (physical mixing Vs co-processing) employed.