ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Ghazala Y. Hermiz Assistant Professor, Department of Physics, Science College, Baghdad University, Iraq |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Samples of Mg-doped bismuth 2223 superconducting materials with nominal composition Bi1.6Pb0.4Sr2Ca2- xMgxCu3O10+δ (0≤ x ≤0.5) were synthesized by solid-state reaction method .The effect of magnesium on structural ,electrical resistivity and dielectric properties [dielectric constants (real and imaginary part ), dielectric loss factor (tan δ) and ac-conductivity (ζ ac) as a function of frequency ] has been investigated . XRD analysis showed the system has an orthorhombic structure with c/a ratio for Bi1.6Pb0.4 Sr2Ca1.8Mg0.2Cu3O10+δ system more than the other samples. A sharp drop of resistivity was observed for the same sample with a transition temperature equal to 104 K. The negative value of capacitance at lower frequencies for samples with x=0, 0.2, 0.5 has been observed in the dielectric measurements of these samples. This may attributed to difference in the Fermi levels of the contact electrodes with the superconductor ceramic sample.
Keywords |
| Mg effect, dielectric properties, ac-conductivity, superconductor |
INTRODUCTION |
| The Bi-Sr-Ca-Cu-0 superconductor which was discovered by Maeda et al. [1] is considered as one of the important materials for device applications. Impurity substitution in the cuprate superconductors has a powerful of the basic electronic properties. For example, Pb doping of Bi-Sr -Ca-Cu –O superconducting systems induces a partially melted liquid phase that eases the diffusion of the element to form the high-Tc phases and to increase their critical temperature[2]. Several alloying elements, such as MgO can be added to improve the electrical properties of polycrystalline 2212, and the addition of MgO was reported to have beneficial effects on the grain connectivity and grain alignment in fully processed samples of Bi2223 and on its critical current value [3]. Lin et al.[4] prepared samples with nominal composition Bi2Sr2Ca2Cu3−xMgxOy (0≤x≤0.7) at 850≈910°C. The samples show similar X-ray diffraction patterns and their phases are composed of Bi2Sr2Ca1Cu2Oyand impurity phases. There are little effect of Mg addition on the temperature of onset transition and zero resistance. The Tc(onset) and Tc(zero) strongly relate to the sintering method. Cross et al.[5] found that the addition of MgO to the system improves the critical current density. Microstructure studies indicate that the addition of MgO is not dissolved in the high Tc phase, instead it disperses. Their results indicated a slight increment of the current density by the addition of MgO which attributed to the increment of the flux pinning although essential weak link of intergrain was not improved.The superconducting properties of YBa2(Cu1- xMgx)3O7- delta (0<or=x<or=0.1; delta <or=0.08) and La1.85Sr0.15(Cu1-xMgx)O4(0≤x≤ 0.07) have been studied by Raffo et al [6].In both systems, an increase of the dopant content induces a contraction of the crystallographic c axis and a reduction of the critical temperature Tc.An onset temperature of 79 K is found for YBCO at the maximum Mg solubility (x =0.025). In LASCO, superconductivity is suppressed at x=0.03. Their results support the hypothesis that the detrimental effects on superconductivity of all non-magnetic dopants are similar, and are linked to the formation of a net magnetic moment in the CuO2 plane. Wei et al. [7] added nanosize MgO particles to Bi2.1Sr1.7CaCu2Ox (Bi-2212) powder in various molar fractions. Bulk samples were made from the MgO/Bi-2212 and heat treated by partial-melt processing. Scanning electron microscopy and energy-dispersive X-ray spectra were used to study the phases present in the samples. The effects of the MgO additions on the flux pinning behavior as a function of magnetic field and temperature were investigated by magnetization hysteresis measurements obtained with a SQUID magnetometer and a 33 T vibrating sample magnetometer. They found that the nanosize MgO addition did not affect Tc of Bi-2212, while significantly expanding the magnetization hysteresis loops and increasing the irreversibility field. X-ray and SEM analysis suggested that MgO is inert with respect to Bi-2212. Nawazish et al.[8] synthesized the Cu0.5Tl0.5Ba2Ca2−y Mg y Cu3O10−δ (y=0, 0.05, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 1.5, 2.0) superconductor at the atmospheric pressure by the solid-state reaction method. The zero resistivity critical temperature is found to increase to 98 K with Mg concentration of y=0.6, but saturates to 97 K with further enhancement of Mg to y=0.8, 1.0, and 1.5. The Mg doped material grows in tetragonal structure with a & c-axes lengths of 3.894 Å & 15.091 Å for y=1.5. The axes lengths were decreased with the increase of Mg content in the unit cell, which shows that anisotropy of the material decreases. The critical current density and the quantity of diamagnetism in the samples with Mg contents are higher than in the samples without Mg.The influence of Ag, Mg, and Pr additions and co-additions on microstructure and phase formation of Bi2Sr2CaCu2O8+d (Bi2212) system was investigated by Boussouf et al.[9]. Polycrystalline Bi2212 samples were synthesized in air by solid state reaction method. Phase analysis, micro structural observations and magnetic properties were carried out by X-ray diffraction (XRD), scanning electron microscopy (SEM) and A.C. susceptibility measurements respectively. XRD results reveal two main phases (Bi-2201 and Bi2212). SEM photographs show that the substitution by Ag, Mg or Pr affects the mechanism of the grains growth. The undoped sample has a critical temperature Tc of 65 K while in the Mg and Ag containing compounds the Tc is 77 K and 75 K respectively. The Pr containing compound exhibits no superconductivity. A valence of the Pr ion higher than 3+ in the lattice supports the hole filling mechanism of the suppression of superconductivity. Kocabas et al.[10] studied the effects of Mg substitution in Bi-2223 superconductor system for the Bi1.7Pb0.3Sr2Ca2Cu3−x Mg x O y nominal composition (x=0.00, 0.05, 0.10, 0.15 and 0.20) prepared by the conventional solid-state reaction. The properties of these compounds were investigated by measuring the electrical resistivity, X-ray diffraction (XRD) and density. Also, the surface microstructure of the samples was studied by SEM. They found that the effects of Mg substitution support the development of both the Bi-2212 and Bi-2223 phases. They observed a decreases of the onset critical temperatures with addition x>0.10. The presence of Mg influenced the microstructure of the samples and decreased the mean grain size of Bi-2223 grains up to x=0.10. Younis et al.[11] studied the dielectric properties of Cu0.5Tl0.5Ba2Ca2−q Mg q Cu3O10−δ (q=0, 0.5, 1.0 1.5) superconductor samples at two temperatures of 80 and 290 K by capacitance (C) and conductance (G) measurements with the test frequency (f) in the range of 10 KHz to 10 MHz . Their measurements include the dielectric constants (ε′ and ε″), dielectric loss factor (tan δ) and ac-conductivity (ζ ac) as a function of frequency and temperature. This work is aimed to produce high temperature superconductor in the Bi-compound, prepared by solid state reaction. The effects of substitution of Mg on Ca in Bi1.6 Pb0.4Sr2Ca2-xMgxCu3O10+δ for (0≤x≤0.5) on the structure, electric and dielectric properties were investigated. |
II. EXPERIMENTAL WORK |
| The samples were prepared by solid state reaction, using appropriate weights of pure materials Bi2O3, Sr(NO3) 2,CaCO3, CuO, Pb3O4 and MgO, and in proportion to their molecular weights. The mixture homogenization takes place by adding a sufficient quantity of 2-propanol to form paste, during the process of grinding for about (50-60) min. The mixture was calcinied in a tube furnace in air that has programmable controller type (Eurptherm818) for 24 hours at 800 °C. This mixture was then pressed into pellets 1.3 cm in diameter and (0.2 – 0.3) cm thick, using hydraulic type (SPECAC) under pressure of 0.7GPa. The pellets placing in alumina crucible then sintered for about 140 hours at 830 °C. The structure of the prepared samples was obtained by using x-ray diffractometer (XRD) (Philips).A computer program was established to calculate the lattice parameters a, b, and c. Four probe dc methods at a temperature range of (80-3000) K were used to measure the electrical resistivity (ρ) and to determine the critical temperature (Tc). The real(ε1) and imaginary(ε2) dielectric constants, absolute dielectric loss | tanδ| and ac-conductivity(ζac) were calculated by measuring the capacitance (C) and conductance (G) with the aid of LCR meter impedance analyzer type (Avcitive Awia;Agilent 4294A) for frequency range 25 KHz to 5MHz.By using the following expressions[12,13]: |
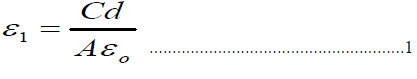 |
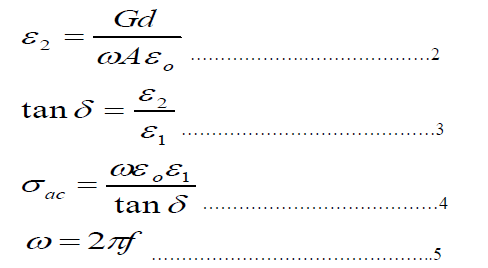 |
| Where d is the thickness of the pellet, A is the area of the electrode, εo is the permittivity of free space and f is the frequency of the applied field. |
III. RESULT AND DISCUSSION |
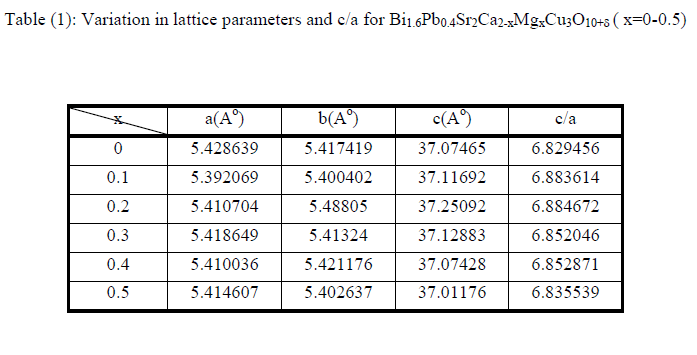
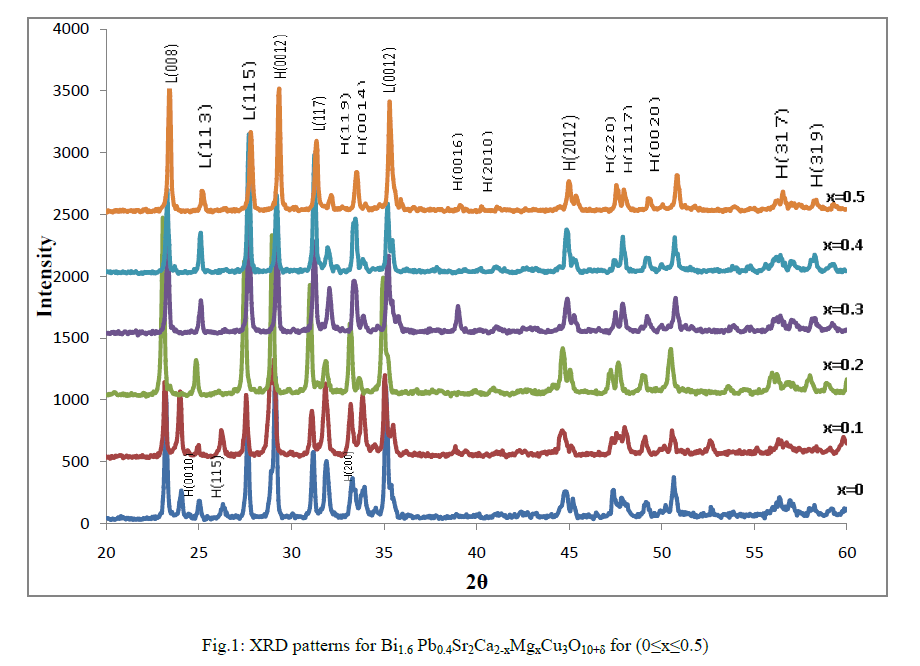
| The XRD measurement were carried out for various samples of Bi1.6 Pb0.4Sr2Ca2-xMgxCu3O10+δ with Mg varying from 0 to 0.5 are shown in Fig.1, the indices in the patterns are after Koyama et al.[14] and Primo et al.[15] .The XRD spectra correspond to high Tc-phase 2223(H) as a dominate phase with low Tc-phase 2212 (L) and impurity phases. The unknown phases could interpret to creation of the Mg compounds or/and to the stacking faults which leads to deform the structure. Another observation has been found that the intensities of the diffraction peaks for the same Miller indices of (hkl) are varied for different composition. Beside of that there is a shifting of peak position in compression with pure sample, this may attributed to the substitution of Mg in Ca sites. The lattice parameter a,b and c ,the ratio c/a were calculated for all samples .It is shown from Table(1) the system has an orthorhombic structure with c/a ratio for Bi1.6Pb0.4 Sr2Ca1.8Mg0.2Cu3O10+δ system more than the other samples which indicates a more isotropic crystal structure of this system . |
 |
 |
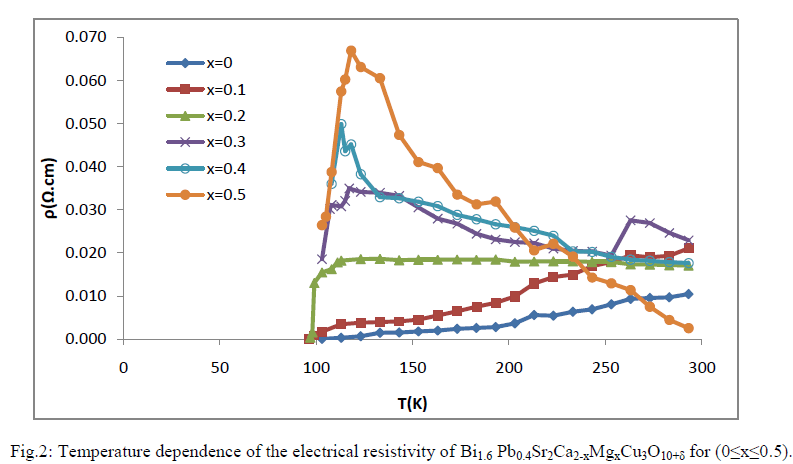
| The temperature dependence of the electrical resistivity of Bi1.6 Pb0.4Sr2Ca2-xMgxCu3O10+δ for (0≤x≤0.5) are shown in Fig.2 .It is noticed that the samples behave as a superconductor with a transition temperature equal to 113,100 and 104 for the composition with x=0,0.1 and 0.2 respectively .A sharp drop of resistivity was observed for the composition Bi1.6Pb0.4Sr2Ca1.8Mg0.2 Cu3O10+δ ,while the superconducting transition were not sharp for magnesium free samples and with x=0.1 .This may be due to fluctuation of oxygen content or to the existence of small amounts of the secondary phase as shown in the XRD pattern. For the other samples with x=0.3,0.4 and 0.5 , they behave like a semiconductor in between 300K to 118K ,after that, it is found a drop in resistivity with the reduction of temperature, but a complete zero resistance could not be observed which means the Tc (off) is less than the boiling point of liquid nitrogen. Another feature was observed from Fig.2 an increasing of the electrical resistivity with increasing of Mg content. The reason may attribute to the poor connected, randomly oriented grains and large number of voids; thus end to end transport connectivity was still poor as referred by Kellner. et al.[16]. |
 |
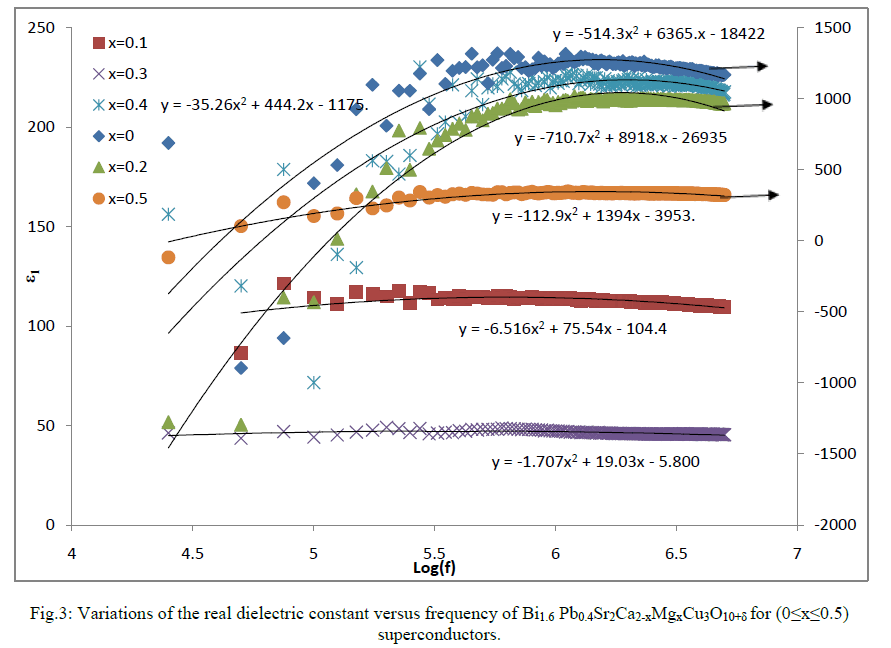
| Fig.3 shows the variation in the real part of dielectric constant (ε1) of Bi1.6 Pb0.4Sr2Ca2-xMgxCu3O10+δ for (0≤x≤0.5) as a function of frequency. The difference of ε1 for samples is due to the changed nature of the material within the conducting planes and to a modification of the carriers there as indicated by Younis et al. [12].The negative value of ε1 at lower frequencies for samples with x=0, 0.2, 0.5 give an indication of a reduction of the capacitance of the sample from the geometric capacitance without the sample. This may attributed to the positive space charges in the neighborhoods of the electrodes. Indeed when the mobile charge carriers within the conducting planes are displaced from their equilibrium positions this will generate a dipolar capacitance. The positive space charge is probably at the device’s outer surface because the free negative charge carriers flow towards the metal electrodes from the ceramic sample. The flow of electrons from the ceramic to the metal surface is possible because the Fermi level of the ceramic is higher than that of metals [17]; the metals have more filled states than ceramics do and the filled states have lower energies than vacant ones. At higher frequencies the magnitudes of ε1 for the samples approach almost the same values; where the time constant of the applied signal becomes much smaller than the time constant of dipolar polarization and the material appears to have the least polarization. |
 |
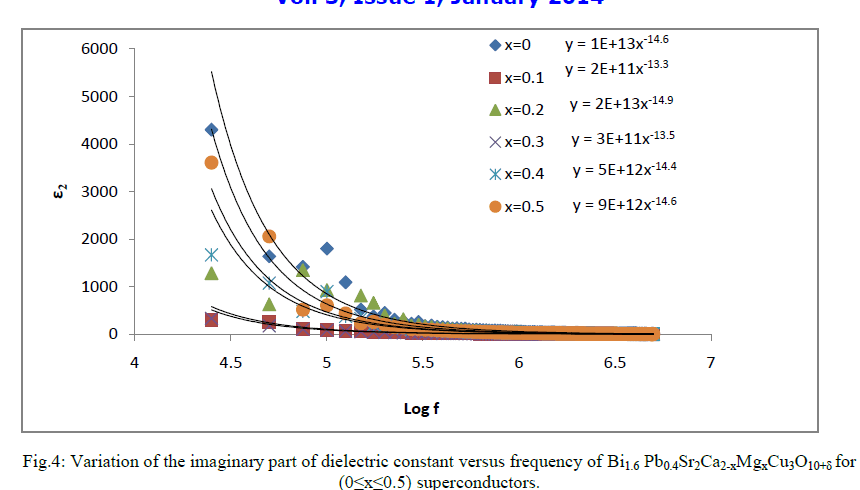
| The value of the imaginary part of dielectric constant (ε2) of Bi1.6 Pb0.4Sr2Ca2-xMgxCu3O10+δ for (0≤x≤0.5) was found to decreases with increasing frequency as shown in Fig.4. The higher values of ε2 for free magnesium samples as compared with doped samples as mentioned from this figure may attributed to the lower conductance of Mg. ε2 emerges as a result of the phase lag of the displacement in response to the applied field. The imaginary part of the dielectric constant refers to the absorption and attenuation of energy across the interfaces (grain boundaries, localized defects, and localized charge densities at the defects sites) under the applied external electric field. |
 |
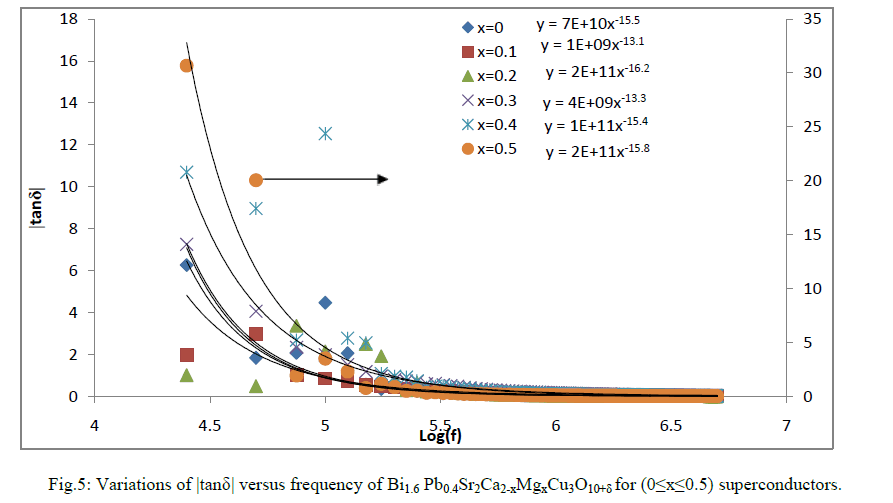
| The ratio of energy dissipated and energy stored in the material determines the dielectric loss factor| tanδ| and the absolute value of dielectric loss factor verses log f for Bi1.6 Pb0.4Sr2Ca2-xMgxCu3O10+δ for (0≤x≤0.5) samples are shown in Fig.5. The values of | tanδ| were decreased with the increase of frequency in all the samples. |
 |
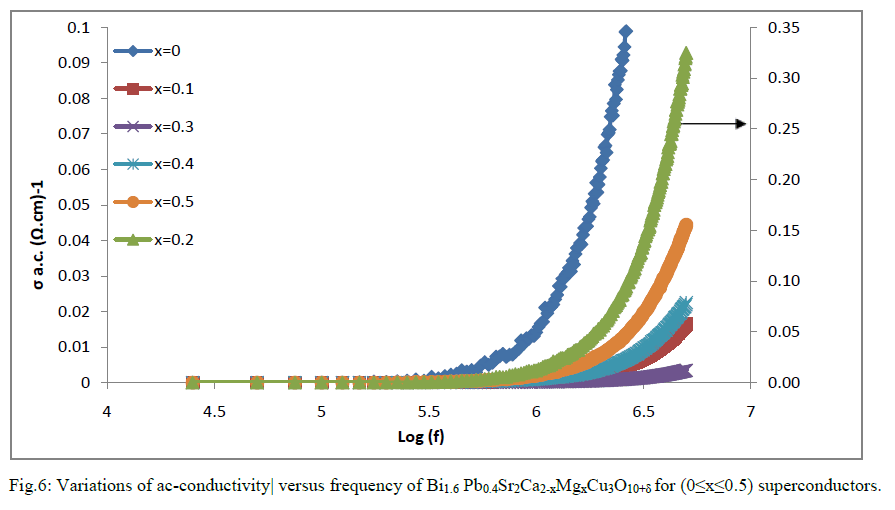
| The ac-conductivity of the Bi1.6 Pb0.4Sr2Ca2-xMgxCu3O10+δ for (0≤x≤0.5) superconductor samples is shown in Fig 6.The values of ζac is remain constant till a certain value of frequencies dependence on the x value this could be explained to the time constant of the applied signal becomes shorter than the time constant of dipolar polarization. After that there is an enhancement of ζac with increasing of frequency .Also it is found the composition Bi1.6 Pb0.4Sr2Ca1.8Mg0.2Cu3O10+δ has a higher value of the ac-conductivity in comparison with the free and Mg-doped samples. That means increases of Mg content up to 0.2 will increases of the carrier concentration. More addition of Mg atoms decreases the conductivity The decrease of the conductivity is related to the localization of charge carriers. Disorder within the grains decreases the effective coupling within the grains by increasing the coulomb interaction [18]. |
 |
IV. CONCLUSION |
| XRD analysis showed the Bi1.6Pb0.4Sr2Ca2-xMgxCu3O10+δ (0≤ x ≤0.5) system has an orthorhombic structure with c/a ratio for Bi1.6Pb0.4 Sr2Ca1.8Mg0.2Cu3O10+δ system more than the other samples. A sharp drop of electrical resistivity was observed for the same sample .The dielectric constants (ε1 and ε2), the dielectric loss |tanδ|, and the ac conductivity (ζac) of the samples are strongly depend on the frequency of the applied ac field. The dielectric dispersion and the negative capacitance effects at lower frequencies for samples with x=0, 0.2, 0.5 has been observed in dielectric studies. |
References |
|