ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
A. Mushtaq Ahmed Khan1, M.Subramanian2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The molecular structure and molecular forces in liquids and solutions in particular have been investigated by dielectric relaxation studies. The nature and strength of the molecular interactions have been established as the main cause for the chemical behavior of compounds. The dielectric behavior of Triethylamine with Dimethyl Phthalate has been studied at microwave frequency 9.36 GHz at different temperatures 303K, 308K and 313K. Different dielectric quantities like dielectric constant (ε′), dielectric loss (ε″), static dielectric constant (ε0 ) and dielectric constant at optical frequency (εα) have been determined. The relaxation time τ has been calculated by both Higasi’s method and Cole-Cole method. The complex system investigated shows the maximum relaxation time values at temperatures by both Higasi’s method and Cole-Cole method.
Keywords |
| Dielectric constant, Dielectric loss, Optical frequency, Higasi’s method, Cole–cole method, Dimethylphthalate, Trimethylamine. |
INTRODUCTION |
| The dielectric relaxation behaviour of mixtures of polar molecules under varying conditions of complexation, temperature and environmental factors has evoked considerable interest. Based on the results, models of relaxation process in liquid mixtures have been formulated. So many researchers [1-6] studied the association of two polar molecules due to hydrogen bonding from the dielectric relaxation measurements at microwave frequencies. Purcell and Smyth [7] were the first to detect solute - solvent interactions through measurements of relaxation time. The dielectric relaxation studies of ternary mixtures of polar solvents in dilute solutions of non-polar liquids provide valuable information about solute-solute and solute-solvent interactions. Molecular association between triethylamine with alcohols in benzene in the microwave region was studied by this dielectric relaxation behaviour by S. Kumar et al [8] using single frequency concentration variation method. |
| In order to provide the experimental data on ternary mixture, dimethyl Phthalate with triethylamine in benzene at various concentrations were studied at microwave region at different temperatures of 303K, 308K and 313K [9]. The study is expected to provide better understanding of the nature of molecular orientation processes. |
II. MATERIALS AND METHODS |
| Dimethyl Phthalate with triethylamine in benzene was used. The molar concentrations of the ternary mixture of the triethylamine with dimethyl Phthalate in benzene are 0.1, 0.2, 0.3, 0.4, 0.5, and 0.6. The measurement of dielectric constant at an angular frequency ε’ and dielectric loss ε” was carried out in the X band microwave frequency at 9.36 GHz. The static dielectric constant ε0 was measured by heterodyne beat [10] method at three different temperatures 303K, 308K and 313K, using a dipole meter operated at 220 volts. The refractive index was measured by Abbe’s refractometer [11]. The errors in the measurements of density and refractive index (nD) are 0.002g/cc and 0.002 respectively. The temperature of all these measurements was maintained at 303K, 308K and 313K using a water circulating thermostat. The density was measured with a 20ml specific gravity bottle. |
III. THEORY |
Higasi Method |
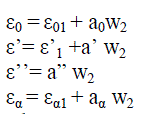 |
IV. RESULTS AND DISCUSSION |
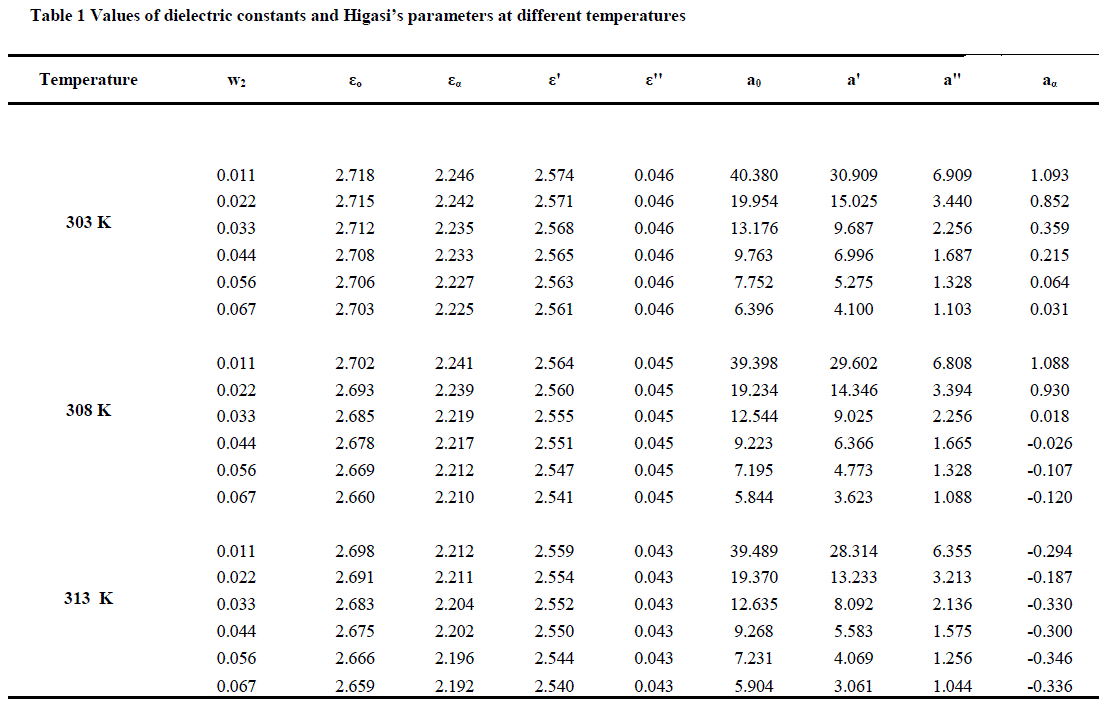
| The values of the dielectric constants at high frequency (ε’), the dielectric loss (ε’’), the distribution parameter (α), the most probable relaxation time (τ), the relaxation time for overall rotation of the molecule (ïÿýïÿý 1 ), the relaxation time for group rotation ( ïÿýïÿý 2 ) and the excess dipole moment for the system benzene + dimethyl phthalate + triethylamine at temperatures 303 k, 308k, and 313k are reported in Table 1. |
| The static dielectric constant, the dielectric constant at optical frequency, the dielectric constant and dielectric loss at microwave frequency decrease with the increase of concentration of the mixture. The variation of the dielectric constant with concentration indicates the hetro junction between the components [15 ]. |
| Higasi’s parameters were calculated using equation (2) and (3). The relaxation time (τ) and the distribution parameter (α) were also determined by the Cole-Cole method using equation (2). Davidson [16] showed that the relaxation process for any system can be resolved into inter molecular relaxation time τ(1), and intra molecular relaxation time τ(2) components only if the ratio of the relaxation time τ(1) τ(2) is greater than 6. In our present investigation, no such resolution is found to occur owing to the increased overlap of the nearby equal regions. The different sizes of the relaxing units give rise to a changed environment, but not to a distinguishable change in the multimeric unit responsible for different relaxation times. Our results are consistent with the interpretation that there is a progressive change in the n-mer and no abrupt change on the dilution. |
 |
| The decrease in τ (1) and τ(2) wit h dilution is assigned to the red uction in t he sizes of the n-mer s due to the intervention caused by the solvent . The coupling between the dipoles is also reduced by the solvent, enabling the dipoles to rotate more freely. Similar results were reported by the Dannhauser et al. [17] and Cambell et al[18]. |
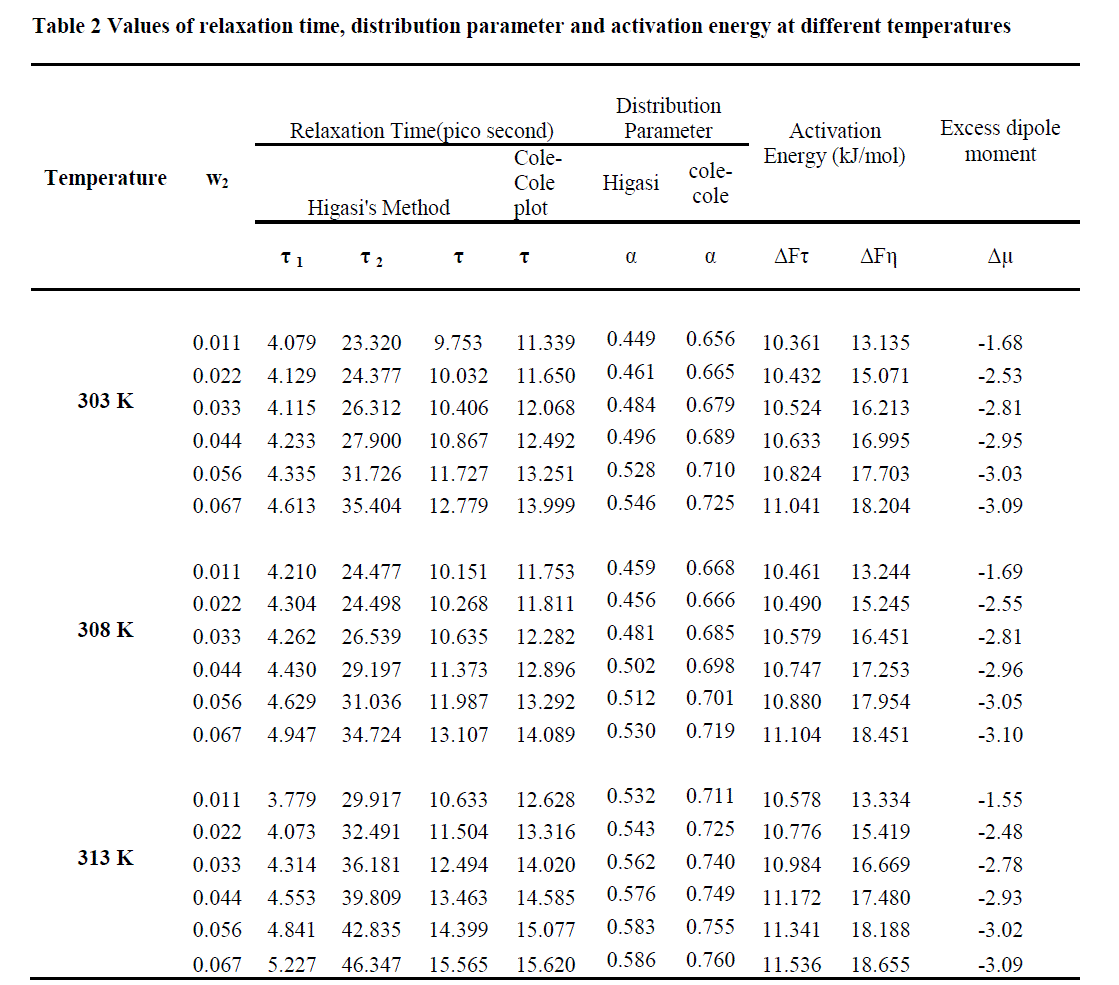 |
| The τ values obtained from the Cole –Cole plot are higher than the values obtained by Higasi’s method. This may be attributed to the rigid behavior of solute molecules. Similar results were reported by S. Krishnan et al. in the studies of alcohols and triethylamine [19]. |
| For all the concentrations and temperatures studied, the free energy of activation (ΔFτ) is less than the corresponding values of the viscous flow (ΔFη). This is in agreement with the fact [20-21] that the process of viscous flow involves greater interference by neighbours than does dielectric relaxation as the latter takes place by rotation only whereas the viscous flow involves both the rotational and translational forms of motion. |
| The excess dipole moment is a qualitative index for the presence of a hydrogen bond in the ternary system. The excess dipole moment of the mixture is calculated using the following equation |
| Δμ= μab - μa - μb (10) |
| The excess dipole moment may be attributed to the proton - transfer in this bond. Similar results were reported by Thenappan [22] for the mixture of amines and alcohols in benzene and by Subramainian [23] for the mixtures of alcohols and nitriles in benzene /1,4 dioxane . The values of relaxation times, distribution parameters, activation free energies and excess dipole moments at various temperatures are reported in Table 2. |
| The values of Δμ are found to be negative for all the concentrations and temperatures . This shows the absence of ionic structures[24, 25]. The negative value of Δμ also indicates the presence of hydrogen bonds between the partners. |
V. ACKNOWLEDGEMENT |
| The authors express their sincere thanks to the College Managing Board of Sree Sevugan Annamalai College, Devakottai and Dr.Zakir Husain College, Ilayangudi for their constant encouragement and providing research facilities. The authors express their heartfelt thanks to the UGC for utilizing the instruments purchased from the Major research funding provided to one of the authors. |
References |
|