ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
S. Sreehari Sastry 1 S.V.Kumara Sastry2, T. Vishwam3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The dielectric behavior of the polar binary mixture of alkyl p-hydroxy benzoates (where alkyl carbon number n varies from 2 to 9), isopropanol for various volume fractions in nonpolar solvent benzene is studied using plunger and cavity perturbation techniques in the microwave frequency range at 10 GHz (X band), HP impedance analyzer at 1 kHz, 10 kHz, 100 kHz, 1 MHz, 10 MHz and Abbe’s refractometer for optical (1015 Hz) frequency region. Hamiltonian quantum mechanical calculations such as AM1, PM3 and MNDO optimized converged geometry operation is performed by using Spartan Modeling software for both pure and binary systems of alkyl p-hydroxy benzoates and isopropanol. Dipole moments of the binary mixtures are calculated from the dielectric data using Higasi’s method and compared with the theoretical results. Conformational analysis of the formation of hydrogen bond between alkyl p-hydroxy benzoate and isopropanol is supported by the FT-IR spectra. The average relaxation times are calculated from their respective Cole–Cole plots.
Keywords |
| Dipole moment, Relaxation time, Hamiltonian calculations, Hydrogen bond, FT-IR. |
INTRODUCTION |
| Dielectric study of liquid mixtures has gained importance [1-3] because it provides one way of bringing together the molecules of different compounds and allows them to interact with one another. The forces responsible for such interactions cannot be studied when such molecules are isolated. Molecular mixtures bring about changes in thermodynamic properties like entropy, free energy and heat of mixing. Changes in physical properties like density, molar volume, refractive index, dielectric constant etc. are manifestations of intermolecular interactions in liquid mixtures [4, 5]. Dielectric investigations of solutions containing varying amounts of interacting molecules help to detect the formation and composition of complexes in them. The nature of complex formation in binary mixtures is still far from clear. The present trend is of liquid mixtures leading to formation of hydrogen bonding in the system due to solute-solvent interactions. Hydrogen bonding is complex in the liquid state because of the uncertainty in identifying the particular bonds and the number of molecules involved. One of the physical parameters used for conformational analysis of any resultant structure is the net dipole moment [6].The study of dielectric dispersion and absorption of a binary liquid mixture provides a very sensitive tool for detecting molecular interactions. It has been observed that the formation of complexes or the presence of association leads to relaxation times considerably higher than those for the uncomplexed (unassociated) species. It has been shown by Schallamach [7] and other workers [8-10] that a binary liquid mixture, in which both components are either associated or non-associated shows a single relaxation time, whereas two distinct relaxation times are observed in the mixtures in which one component is associated and the other is non-associated. Dielectric dispersion studies of polar liquids such as alcohols, benzoates and their binary mixtures were carried out to determine the nature of interaction that exist between the molecules due to hydrogen bonding dipole moment and the relaxation behavior phenomena. Dielectric spectroscopy is sensitive to changes in bonding between different species of liquids in a liquid-liquid binary or tertiary systems-even to weak hydrogen bonding. Dipole moments are determined for the polar solute system diluted in non-polar solvent benzene to minimize the dipole-dipole interaction. The dielectric behavior of some hydrogen bonded polar liquid mixtures was studied by Vishwam [11]. The liquid mixtures in the present study are useful for hydrogen bonding formation with liquid crystals [12] which eventually affect the dipole moment and relaxation time. The relaxation time studies are carried out [13] in the binary mixtures of isopropanol with methyl/ethyl benzoates. The present paper is aimed at studying the dielectric behavior of binary mixture of alkyl p-hydroxy benzoates, which are used in various fields viz. food preservatives, cosmetic preservatives[14-16], and isopropanol (solute system) by varying its concentration in non-polar solvent medium benzene in the microwave frequency range at 10 GHz (X-band) by using plunger and cavity perturbation [3,17,18] methods. Dipole moment values are calculated using the Higasi’s method [19] and compared with the theoretical Hamiltonian quantum mechanical calculations [20, 21]. Minimum energy structure of these polar systems alkyl p-hydroxy benzoates, isopropanol and their equimolar binary system are determined from the AM1, PM3 and MNDO converged geometry optimization procedure using Spartan Modeling software [22]. Conformational analysis of the formation of hydrogen bond between the above systems is carried out from FT-IR spectra in the 450-4000 cm -1 region. The average relaxation times are calculated from their respective Cole-Cole [23] plots. |
EXPERIMENTAL |
| Alkyl p-hydroxy benzoates were procured from M/s Frinton Laboratories, Inc., USA. Isopropanol (IPA) and benzene were Qualigen chemicals of analar grade and double distilled before use. The binary mixture of alkyl p-hydroxy benzoates and isopropanol is used as solute and benzene as non-polar solvent in the preparation of solution. First alkyl p-hydroxy benzoates (nHB) were dissolved in isopropanol (IPA) at various mole fractions (MF) i.e., 0.02%, 0.03%, 0.04% and then each concentration of solute was diluted with solvent in different volume fractions i.e., 0.1, 0.2, 0.3 and 0.4. All the weights were measured using a single pan electronic balance Dhona make, model 100 DS with an accuracy of 0.01 mg. |
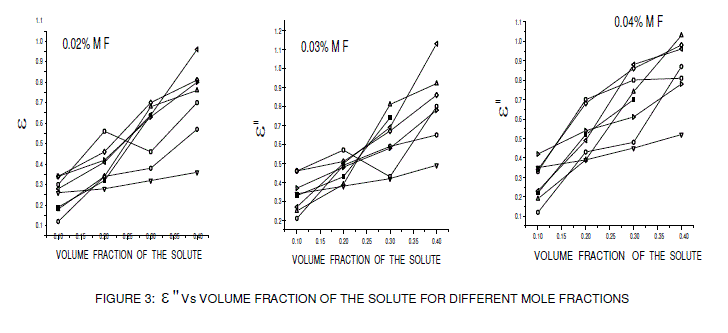
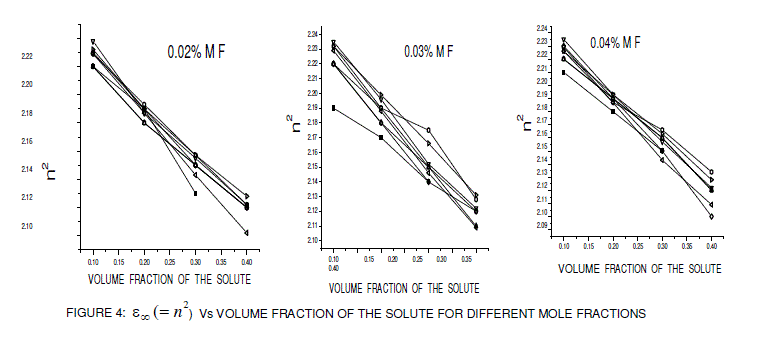
| The low frequency dielectric permittivity ( static ε ) values of the above systems were measured at using a digital LCR meter at room temperature and their variation with volume fraction is shown in figure 1. The dielectric data using HP impedance analyzer was measured at spot frequencies of 1 kHz 10 kHz, 100 kHz, 1 MHz and 10 MHz. The real (ε Ãâó) and imaginary (ε ÃâóÃâó ) parts of the complex dielectric permittivity ( * ε =ε' -jε" ) were determined at room temperature at a frequency 10 GHz (X band) using plunger and cavity perturbation techniques, whose variations with volume fraction are shown in figures 2 and 3. The main advantage of cavity perturbation technique is that a small volume of sample is required i.e., 2ml and it is very useful for low-loss samples, where as plunger technique is useful for high-lossy compounds but it requires large amount of sample i.e, 80 ml. The refractive index of the dilute solution was determined using M/s ASCO make Abbe refractometer with sodium D light as source (1015 Hz) at room temperature. The high frequency dielectric constant ( ε ∞ =n2 ) was obtained from the refractometry measurements and their variations with volume fraction are shown in figure 4. The error in estimation of the low frequency dielectric constant ( static ε ), the real part of dielectric permittivity (ε ' ) and refractive index was 1%. The error in the estimation of relaxation time ( τ ) and dipole moment (μ ) was 2% and for the imaginary dielectric permittivity (ε ") was 3%. The formation of hydrogen bond in the solute system was studied using FT-IR spectra in the 450-4000 cm-1 region with a Perkin Elmer BXF1- FT-IR spectrometer. |
DETERMINATION OF PARAMETERS |
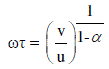 |
RESULTS AND DISCUSSION |
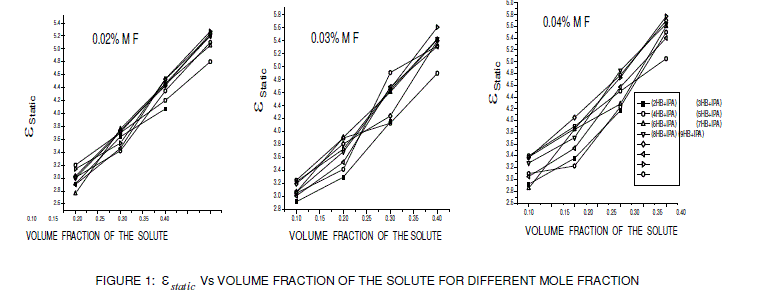
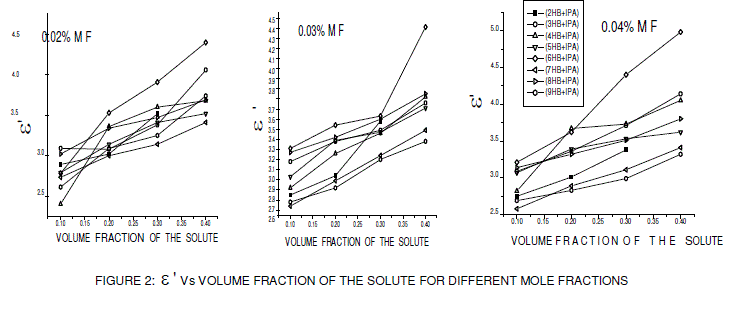
| From the figures 1, 2 and 3 it is observed that the static dielectric constant, real part of dielectric permittivity (ε Ãâó) and imaginary part of dielectric permittivity (ε ÃâóÃâó ) increased with the increase in volume fraction of the solute, but the optic dielectric constant (figure 4) decreases with the increase in volume fraction of the solute for various mole fractions. |
 |
 |
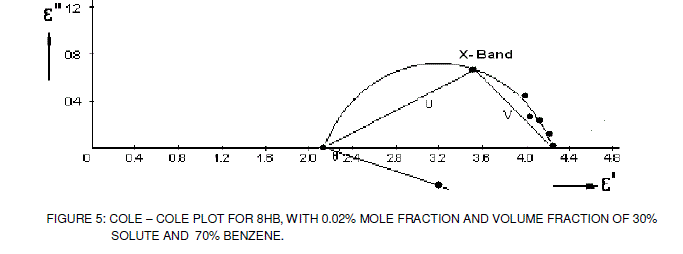
| The relaxation times obtained from Cole-Cole plot is shown in figure. 5 and the data is given in Table 1. The relaxation times are found to increase in magnitude for a given mole fraction of alkyl p-hydroxy benzoates in isopropyl alcohol with the increase of solute volume fraction in benzene which shows that the molecules are unable to rotate freely as benzene concentration decreases in the solution. In general the magnitude of average relaxation time is increasing with the increase of n value in alkyl p-hydroxy benzoate series, which may be attributed to the formation of hydrogen bonding between the solute systems. |
 |
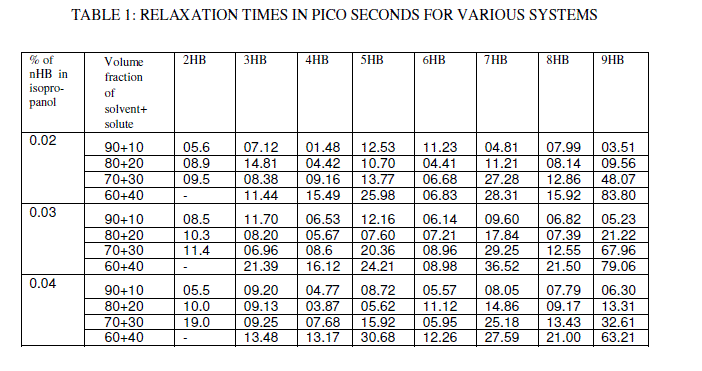 |
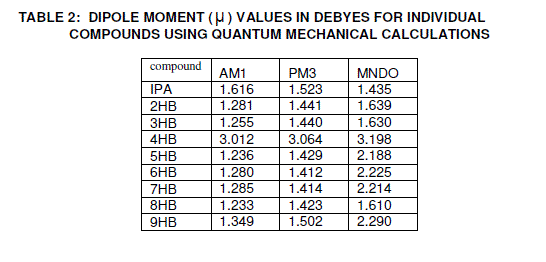
| The dipole moments for various n values of alkyl p-hydroxy benzoate series are shown in figure 6. It is observed that, in general the dipole moment values are increasing with the increase of n value in alkyl p-hydroxy benzoate series. The dipole moments obtained and calculated using Hamiltonian quantum mechanical calculations of AM1, |
 |
| PM3 an MNDO are shown in Tables 2 and 3 along with the average experimental dipole moment values of different mole fractions (0.02%, 0.03% and 0.04%) for the alkyl p-hydroxy benzoate series. Interestingly, the dipole moments show alternation along the series with even numbers having higher dipole moments than the odd neighbours. Thus, it appears that increased side-chain interactions in the odd compounds lead to disturbed bond |
 |
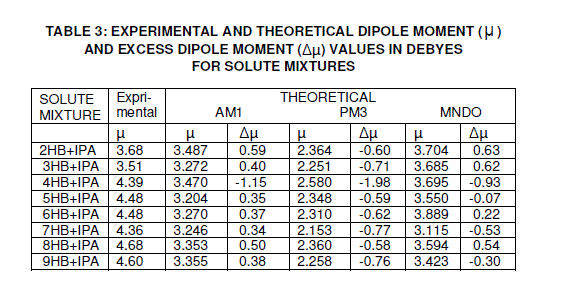 |
| dipoles thereby decreasing the net dipole moment [24]. The theoretical values of dipole moments for individual compounds and solute mixtures are given in tables 2 and 3. It is very clear that there is an increase in the dipole moment values of solute mixtures when compared to the individual systems. This may be due to the formation of hydrogen bonding between the solute systems. The theoretical dipole moment values for the solute systems are in good agreement with the experimental values including the even-odd effect. The small deviation between the theoretical and experimental values may be due to the assumption in calculating the dipole moment of the solute mixture i.e., for theoretical calculations equimolar concentrations are used and for that of experimental different mole fractions are used. The other reason for the deviation between the theoretical and experimental values may be due to electron cloud of non-polar solvent benzene affecting the dipole moment value of the solute system in experimental case [11, 25, and 26]. The experimental and theoretical dipole moment values increase considerably for 4HB and its binary mixture when compared to the remaining nHB and their binary mixtures. The increment in the dipole moment values in the series is mainly due to the contribution of CH2 group in the chain to the dipole moment and this effect is comparatively high in case of 4HB[27-29]. |
| Observing the FT-IR spectra for the solute mixture of (8HB+IPA), there is a shift of≈ 80 cm-1 wave number in the position of –OH for the mixture compared with the pure spectrum of 8HB and a shift of ≈ 110 cm-1 wave number for the mixture compared with the pure spectrum of IPA. This shift is caused by the strong interaction between the high electro-negative charge of oxygen in the benzoate group and the hydrogen of the isopropanol. Thus The IR analysis convinces intermolecular hydrogen bonding of the binary mixture (8HB+IPA) effectively with proportionate variations in stretching frequencies of -OH compared to the pure systems. The experimental and theoretical FT-IR values, which are in good agreement, for –OH group of pure systems IPA, 8HB and solute mixture (IPA+8HB) are shown in Table 4. Optimized geometrical structure of the formation of hydrogen bonding between isopropanol and 8HB is shown in figure 7, which is obtained by Hamiltonian quantum mechanical calculation using Spartan Modeling software. Further, it is observed from the quantum mechanical calculations (AM1, PM3 and MNDO) that the excess dipole moment ( Δμ ) is very low and negative which indicates the absence of any contribution from the ionic structure or charge transfer of the binary system to the total dipole moment. Because if charge transfer effect exists, then Δμ |
| would have been greater than 10D [23, 30, 31]. But in none of the above systems a high value of Δμ is observed. The excess dipole moment value is a qualitative index for the presence of a hydrogen bond between nHB and IPA. The excessive dipole moment is attributed to the proton transfer in the bond. |
 |
CONCLUSION |
| In this paper, the dielectric properties of hydrogen bonded complexes formed by alkyl p-hydroxy benzoates with isopropanol in benzene have been studied by computing relaxation times, dipole moments experimentally and theoretically for various concentrations at room temperature. The formation of hydrogen bond between alkyl p-hydroxy benzoates and isopropanol, which causes an increase in dipole moment values when compared to that of individual systems and also considerable changes in relaxation times, is confirmed by FT-IR spectra. The experimental dipole moment values are in good agreement with the theoretical quantum mechanical calculations. The absence of ionic contribution to the structure is confirmed by the excess dipole moment values. |
ACKNOWLEDGEMENT |
| The authors gratefully acknowledge the University Grants Commission, New Delhi for providing financial assistance through the Major Research Project No. F: 34-12\2008 (SR) dated 30-12-2008. and UGC DRS LEVEL III program No.F.530/1/DRS/2009 (SAP-I), dated 09-02-2009 New Delhi, to the department of Physics. |
References |
|