e-ISSN: 2319-9849
e-ISSN: 2319-9849
Department of Chemistry, RD National College, Linking Road, Bandra (W), Mumbai – 400050, Maharashtra, India
Received date: 20/05/2013; Revised date: 27/07/2013; Accepted date: 04/08/2013
Visit for more related articles at Research & Reviews: Journal of Chemistry
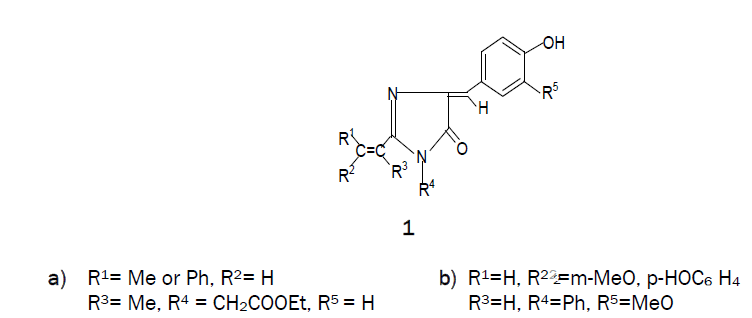
Condensation reactions of N-acetyl-, N-benzoyl and N-ptolylglycines with vanillin in presence of isothiocyanate gives 2-m-methoxyp- hydroxystyryl-, 2-p-tolyl- and 2-phenyl -4-m-methoxy- phydroxybenzylidene- 1-phenyl-2-imidazolin-5-ones respectively. These compounds were evaluated in vitro for antibacterial activity.
5-Imidazolones, 5- Oxazolones, chromophore, antibacterial activity.
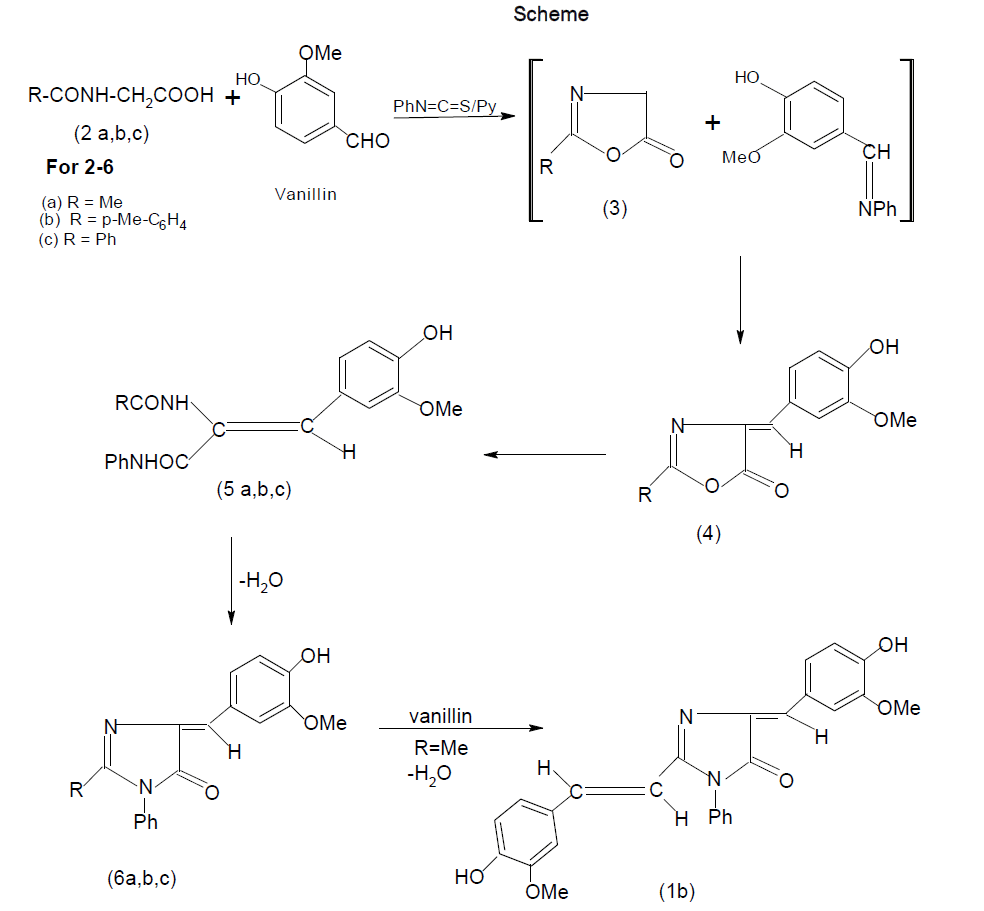
Imidazolines are useful intermediates in natural products synthesis as well as common building blocks found in many biologically active molecules. They are reported to have remarkable pharmacological profile [1-3] including anticancer [4], anti-HIV agents [5], anticonvulsant [6,7] mono-aminooxidase (MAO) inhibitory [8], antimicrobial [9-12] etc These imidazoles are also important because of its application in polymer chemistry [13,14]. 1-Methyl-2- vinyl-1H-Imidazole is the monomer generally used for the preparation of interesting imidazole contatining polymers [15]. 1, 2-Disubstituted-p-hydroxybenzylidene-2-imidazolin-5-ones (1a) were implicated as the chromophore responsible for fluorescence of the green fluorescent protein of Aequorea and Renilla, and their synthesis involved a circuitous route [16]. In our approach to construct a similar system, N-acetylglycine, N-p-tolylglycine and N-benzoylglycine were heated with phenyl isothiocyanate in the presence of vanillin, using pyridine as a catalyst. Construction of a somewhat complex organic molecule generally involves several steps. In some cases, the steps can be telescoped and the synthesis can be accomplished in one flask. These and similar other transformations designed to give a desired product can be called ‘disciplined reactions’. The concept is illustrated in this communication by the synthesis of the title compounds.
Experimental
All chemicals used were of LR grade and the melting points reported were taken in an open capillary tube and are uncorrected. All yields refer to isolated product after purification. The products were confirmed by IR spectra. The 1H NMR of the samples was also recorded.
General Procedures
2-m-Methoxy -p-hydroxystyryl-4-m-methoxy-p-hydroxybezylidene-1-phenyl-2-imidazolin-5-one (1b): A mixture of N-acetylglycine, phenyl isothiocyanate and vanillin taken in the ratio 1: 1.2: 2 respectively, was heated for 30 min at 160-70o with pyridine as a catalyst. The residue was washed with light petroleum (b.p. 40-60°), sodium bicarbonate solution and water, and the products were separated by fractional crystallization from benzene and/or ethanol. The yield was 41%, m.p. 225° (Found: C, 69.21; H, 5.63; N, 5.92. C26H22N2O5.1/2H2O requires: C,69.18; H, 5.09; N, 6.20%); Vmax 3250 and 3150 (OH), 1170 (CO), 1660 and 1640 cm-1 (C=C); d (DMSO-d6) 3.80 (3H, s, OCH3) 3.90 (3H, s, OCH3), 6.30 (1H, d, J 16 Hz, ArCH =CH), 6.75-7.75 (14H two exchangeable, m, 11 x Ar-H, =CH, and 2 x Ar-OH) and 7.90 (1H, d, J 16Hz, ArCH=CH).
2-p-Tolyl -4-m-methoxy-p-hydroxybenzylidene -2-oxazolin-5-one (4b): It was prepared by the procedure previously reported [6] and recrystallised, from ethanol, (35%), m.p. 172-73° (Found: C, 69.72; H, 5.01; N, 4.53%); Vmax 3400 (OH), 1780, 1770 (azlactone) and 1650 cm-1 (C=C); d(CDCl3) 2.41 (3H, s, ArCH3), 6.14 (1H, s, exchangeable, ArOH) and 6.87-8.87 (8H, m, ArH and =CH).
2-N-p-Tolylamino-m-methoxy-p-hydroxy-cinnamanilide (5b): It was prepared by the procedure previously reported [7] and recrystallised from ethanol. The yield was 33%, m.p. 200°C (Found: C, 69.72; H, 5.01; N, 4.71. requires: C, 69.90; H, 4.85; N, 4.53%); IR: (nujol) 3320 (OH), 3290 and 3200 (NH), 1660cm-1 (CO and C=C). Because of insufficient solubility, its 1H NMR spectrum could not be recorded.
2-p-Tolyl-4-m-methoxy-p-hydroxybenzylidene -1-phenyl-2-imidazolin-5-one (6b): The procedure is same as given under compound 1b. The yield was 48%, m.p. 230° (Found: C, 71.21; H, 5.35; N, 6.92. requires: C, 71.64; H, 5.47; N, 6.96%); IR: (nujol) 3400, 3200 (OH), 1720 (CO), 1640 cm-1 (C=C); PMR: d(CDCl3) 2.31 (3H, s, ArCH3), 3.26 (3H, s, OCH3), 5.81 (1H, s, exchangeable, ArOH) and 6.74-7.94 (13H, m, 12xArH and =CH).
2-Phenyl-4-m-methoxy-p-hydroxybenzylidene -1-phenyl-2-imidazolin-5-one (6c): The procedure is same as given under compound 1b. The yield was 58.90%, m.p. 120° (Found: C, 73.23; H, 5.38; N, 6.89. requires: C, 74.23; H, 5.47; N, 6.96%); IR: (nujol) 3400, 3200 (OH), 1720 (CO), 1640 cm-1 (C=C); PMR: d(CDCl3) 2.31 (3H, s, ArCH3), 3.26 (3H, s, OCH3), 5.81 (1H, s, exchangeable, ArOH) and 6.74-7.94 (13H, m, 12xArH and =CH).
When N-acetylglycine was heated with phenyl isothiocyanate at 160-1700C in the presence of vanillin using pyridine as a catalyst, 2-m-methoxy -p-hydroxystyryl-4-m-methoxy-p-hydroxybezylidene-1-phenyl-2-imidazolin-5-one (1b) was formed and it was found that the yield of 1b increased on increasing the molar ratio of vanillin. Similarly, condensation of p-tolylglycine (2b) and N-benzoylglycine (2c) with phenyl isothiocyanate in the presence of vanillin led to the formation of a mixture from which 2-p-tolyl-4-m-methoxy-p-hydroxybenzylidene-1-phenyl-2-imidazolin-5-one (6b) and 2-phenyl-4-m-methoxy-p-hydroxybenzylidene-1-phenyl-2-imidazolin-5-one (6c) was isolated as the main product. Also, small amounts of corresponding 2-p-tolyl/ 2-phenyl -4-m-methoxy-p-hydroxybenzylidene -2-oxazolin-5-one (4b & 4c) and 2-N-p-tolyl / 2-phenylamino-m-methoxy-p-hydroxy-cinnamanilides (5b & 5c) were obtained in this reaction. Compounds 5b & 5c underwent cyclisation to 6b & 6c on heating at an elevated temperature or in glacial acetic acid in the presence of freshly fused sodium acetate.
The interaction of phenyl isothiocyanate and an N-acylglycine would produce 2-substituited-2-oxazolin-5-one (3) and aniline, both of which would compete for condensation with vanillin when it is present in the reaction. Compound 3 undergoes facile aminolysis, but in the present case N-acylglycylanilides (RCONHCH2-CONHPh) were not discernible, which could be best explained by the assumption that the liberated aniline was converted into vanillin anil, the latter then participated in the condensation with 3 to give 2-substituited-4-m-methoxy-p-hydroxybenzylidene-2-oxalin-5-one (4) and aniline which, in turn, underwent subsequent changes. This mechanism is substantiated by the isolation of 4 and 5 which were prepared by an unambiguous route [17]. It is noteworthy that the methyl group of 6a was found to be active which accounts for the formation of 1b. In contrast, the methyl group of the p-tolyl substituent in 6b was inactive and it failed to undergo condensation with an aromatic aldehyde under the present reaction conditions with an aromatic aldehyde under the present reaction conditions. In the present set of transformations, phenyl isothiocyanate played the role of a counter-attack reagent, all the steps proceeded in a disciplined manner, thereby providing a one-flask synthesis of the target molecule. By alternate route, cyclisation of 2 would give 2-methyl-2-oxazoline-5one 3 by using ethyl chloroformate in the presence of triethylamine in dry benzene which would subsequently condense with aromatic aldehydes or Schiff bases, leading to the formation of 1b. Purity of the compounds was checked by TLC. Products were characterized by spectral data and elemental analyses.
The screening results indicate that all compounds were found to moderately active. It has been observed that compounds 1b showed highest activity against E.coli and S.paratyphi-B strain. On the basis of biological activity results, it may be concluded that the introduction of -OH & -OCH3 groups to the heterocyclic frame work enhanced antibacterial activitiy.
Antibacterial activity
All the synthesized compounds were screened for antimicrobial activity at, 50,100 and 200 μg/ml concentrations against the bacterial strains: against S. aureus , B. subtilis [Gram-positive bacteria] and E. coli , S. paratyphi-B [Gram-negative bacteria] by using agar diffusion method using standard literature protocol [18-20]. Known antibiotic Ciprofloxacin drug was used as standards for comparison of antibacterial activity under the similar conditions. Dimethyl sulfoxide was used as a solvent to prepare the stock solution of the test compounds. The drug was allowed to diffuse for about 4 h into the agar medium before adding the suspension of the test bacteria The plates were incubated at 37 ºC for 48 h and the results were recorded. The tests were carried out in duplicate. The zones of inhibition of the microbial growth produced by different concentration of test compounds were measured in millimeters (mm). The result of this study is given in Table 1.
We are thankful to the authorities of R.D. National and W.A. Science College for providing us with the necessary facilities. Our thanks to the IIT, Mumbai for providing us with the NMR and IR spectra.