e-ISSN: 2319-9849
e-ISSN: 2319-9849
Department of Chemistry, Lovely Professional University, Phagwara, Punjab, India
Received Date: 16/03/2017 Accepted Date: 04/03/2017 Published Date: 08/03/2017
Visit for more related articles at Research & Reviews: Journal of Chemistry
By means of polarization measurements UV, IR, and weight loss study, it has been detected that the extract of Asparagus racemosus leaves can act as corrosion inhibitor for the sake of mild steel in 8% H2SO4 solution. These plants show good inhibition efficiency at particular concentrations of the acid. It is observed that plants extract act as better inhibitor on increasing their concentration. Here we have focused on the corrosion inhibition action of different plant extracts in H2SO4 medium.
Asparagus racemosus, Mild steel, Weight loss, SEM, Polarization measurement
From last few decades, corrosion inhibitor has been a colorable field of research. Acids are being used widely in the field of mild steel machinery in industries for different applications for e.g. cleaning, descaling, pickling etc. [1]. To prevent the corrosion of mild steel by using plant extracts is one of the most enterprising methods. Several organic inhibitors are dynamically used to prevent corrosion and it is the most provident method. The inhibition action of organic inhibitors relies on their adsorption capability on metal by replacing water molecules [2]. Organic compounds also show corrosion inhibition efficiency and there are several organic compounds which are notified to prevent corrosion [3-7]. But the problem with them is that they are extremely poisonous to both humanitarian as well as surroundings. Because of the poisonous effects of these organic inhibitors, natural nontoxic inhibitors are the requirement of our environment [1]. Compounds which contain oxygen and nitrogen inhibit the corrosion of mild steel most effectually [8]. In green inhibitors, secondary metabolize O and N are usually present and they are the active centers for adsorption [9,10]. The adsorption of green inhibitors on the steel surface can be either in the form of physisorption or chemisorptions or it may also be as a combined effect of both [11]. Therefore, to overcome the toxic effect of commercial inhibitors, the development of natural nontoxic inhibitors to prevent the metallic corrosion is necessary and seductive [12]. Extract of naturalistic products contain many compounds which are biodegradable in nature. Here we have used the extracts of Asparagus racemosus with H2SO4 which play an effective role to prevent corrosion of mild steel. The extract of Asparagus racemosus consists of Saponins alkaloid (Figure 1). The inhibition effect of this plant extract on the corrosion rate of mild steel in 8% H2SO4 was studied by using weight loss study, IR, UV and polarization measurements.
Preparation of Plant Extract
The very first, we collected the plants mentioned above for our study, then dried and powered. The powder was soaked into 500 ml deionized water and then refluxed for 5 h. In this manner, we obtained the aqueous solution which was then filtered and concentrated to 100 ml with the help of soxhlet apparatus. Then we prepared the solutions of particular concentrations with the help of this concentrated solution.
4.2. Weight Loss Measurements
With the help of weight loss study, we measured the loss in weight of mild steel strips when they were placed in saturated solution of H2SO4 with extract. For this purpose, we took rectangular pieces of mild steel which composed of (wt %) Fe 97.60%, C 0.083%, Si 0.39%, Mn 0.43%, P 0.12%, Cr 0.45%, Ni 0.27% and Cu 0.43% [13]. These strips were then squeezed with fine grade emery paper (120, 600, 800, and 1000), washed with distilled water and dried. Then the mild steel strips were weighted accurately before inserting them into the saturated solution of H2SO4 with extract. The mild steel strips were then dipped into H2SO4 without extract and saturated with varied concentrations of extract. Then we left the set up for 5 hours and after the elapsed time, the mild steel strips were excluded, cleansed, drained and weighed. A difference in weight was noted between the mild steel strips dipped in H2SO4 solution without extract and with varied concentrations of extract. Here we have used 8% H2SO4 in the experiment. All the concentrations of inhibitors for weight loss are taken in mgL-1 by weight. The inhibition efficiency and surface coverage (θ) can be determined by using following equation:
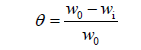 (1)
(1)
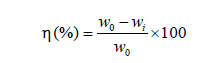 (2)
(2)
Where, wi and w0 are the weight loss values in presence and absence of inhibitor, respectively.
Electrochemical Measurements
Electrochemical studies were conducted by using a Gamry interface Potentiostat/Galvanostate/ZRA0300. All techniques were useful. Polarization with Potentiostat measures current while on the other hand polarization with Galvanostate measures potential. The corrosion cell consists of three electrodes. We used saturated calomel electrode in the form of reference electrode, a platinum foil in the form of counter electrode and mild steel was used in the form of a working electrode. Here, we immersed the working electrode in the test solution and stabilized it for 30 minutes. With the help of such studies, we obtained Tafel polarization curves which helped us to get information about corrosion inhibition of mild steel. These slopes provided us the value of corrosion current densities ( Icorr), we used this value in the following formula and found the inhibition efficiency.
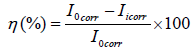 (3)
(3)
Where Iicorr and Iicorr represent the corrosion current density values without and with inhibitor, respectively.
UV-Visible Spectroscopy
We subjected the 8% H2SO4 solution saturated with extract before and after dipping the mild steel strips to UV-Visible spectrophotometer. We obtained the UV-visible absorption spectra for various concentrations of extracts before and after dipping the mild steel strips. The spectra of extract before dipping the mild steel strip showed some smaller peaks which were not found in the spectra of extract after dipping the mild steel strip. It means that when we dip mild steel strip in the extract, some molecules get adsorbed on the mild steel surface. They form a protective film on the surface and prevent the corrosion of mild steel.
IR Spectroscopy
The IR spectroscopy tells us about different types of functional groups, heteroatoms present in the extract.
Surface Analysis
The SEM micrographs of mild steel samples were immersed in 8% H2SO4 solution for 3 h in the absence and presence of extract. A rough surface can be seen for the mild steel immersed in H2SO4 without inhibitor which indicates the corrosion on mild steel surface in acidic medium. In the presence of inhibitor, a smooth surface can be observed, which indicates that inhibitor covers the mild steel surface.
IR Study
IR study tells about the functional groups present in the extract. From the IR spectra of Asparagus racemosus, the peak at 3454 cm-1 suggests O-H stretching of alcohol, the peak at 1639 cm-1 suggests C=O stretching of amide. Further peak at 1057 cm-1 suggests C-O stretching of alcohol. The IR spectr of Asparagus racemosus are shown in Figure 2.
UV-Visible Spectroscopy
The UV spectra of Asparagus racemosus extract saturated with 8% H2SO4 before and after dipping the mild steel strips are shown in the Figures 3 and 4.
Weight Loss Study
For mild steel, the weight loss results in the absence and presence of different concentration of Asparagus racemosus extract saturated with H2SO4are summarized in Table 1. The Table 1 clearly indicates that when concentrations of the Murraya koenigii extract increases, the inhibition efficiency also enhances. The Asparagus racemosus extract gives maximum inhibition efficiency of 77% at 300 ppm. Asparagus racemosus consist of heteroatoms (O, N) which form the metal complex bond with the metal surface thereby reducing corrosion.
| Acid solution | Inhibitor concentration (mgL-1) | Weight loss (mgcm-2) | h (%) | ÃÆÃâÃâÃâÃÆââ¬Å¡ÃâáÃÆÃâÃâââ¬Å¡ÃÆââ¬Å¡ÃâöÃÆÃâÃâââ¬Å¡ÃÆââ¬Å¡Ãâÿ |
|---|---|---|---|---|
| 8% H2SO4 | 0 | 36.06 | 0 | 0 |
| 60 | 11.23 | 68.87 | 0.6887 | |
| 90 | 9.87 | 72.63 | 0.7263 | |
| 120 | 9.23 | 74.4 | 0.744 | |
| 180 | 8.85 | 75.45 | 0.7545 | |
| 240 | 8.42 | 76.65 | 0.7665 | |
| 300 | 8.14 | 77.43 | 0.7743 |
Table 1. Corrosion parameters for mild steel in 8% H2SO4 without and with various concentrations of Asparagus racemosus.
Polarization Measurements
Weight loss study was verified by polarization measurement. The polarization measurement for Asparagus racemosus provided the values of corrosion current density ( I0corr ) which has been shown in Table 2.
| Con.(ppm) | Ecorr(V) | Icorr(A) | Betaa(V/decade) | Betab(V/decade) | CR (mpy) | E% |
|---|---|---|---|---|---|---|
| 0 | -0.44 | 0.0355 | 0.2742 | 0.7565 | 20687 | - |
| 30 | -0.42 | 0.0159 | 0.1469 | 0.3335 | 9237 | 55 |
| 90 | -0.42 | 0.0095 | 0.1202 | 0.2987 | 5545 | 73 |
| 180 | -0.42 | 0.0084 | 0.1216 | 0.2936 | 4916 | 76 |
| 300 | -0.42 | 0.0079 | 0.1092 | 0.2647 | 4585 | 78 |
Table 2. Potentiodynamic polarization parameters for the corrosion of mild steel in 8% H2SO4 without and with different concentrations of extract.
We used these values in the following formula and will find inhibition efficiency.
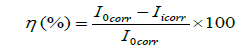 (4)
(4)
Where Iicorr and Iicorr represents the corrosion current density values without and with inhibitor, respectively. Figures 5 and 6 represent the polarization curves of mild steel in 8% H2SO4 without and with various concentrations of Asparagus racemosus extract respectively.
Surface Analysis
The Figure 7 shows the SEM micrographs of mild steel strips immersed in 8% H2SO4 without and with Asparagus racemosus inhibitor respectively.
In the present study, the plant extracts which we used to determine the corrosion inhibition action of plant extracts for mild steel, contain alkaloids. These alkaloids are rich in heteroatoms like N, O. From the IR study of plant extracts, it has been shown that they contain functional groups like hydroxyl group, amide group, carbonyl group etc. Hence, the IR study also verifies the presence of heteroatoms N, O in the extract. These heteroatoms donate their lone pair of electron to the empty d-orbital of Fe. Similarly, there is also the interaction between the Π electrons of C=O and empty d-orbital of Fe. In such a way, the inhibitor gets adsorbed on the mild steel surface and forms a protective thin film by the combination between inhibitor and mild steel surface. This protective film prevents the corrosion of mild steel (Figure 8).
In the present study, we have used the plant extracts of Asparagus racemosus, Tinospora Cardifolia and Voila odorata. We have determined the corrosion inhibition rate of plant extracts for mild steel in 8% H2SO4. The examined plant extract prevent the corrosion of mild steel in 8% H2SO4 by adsorbing on the mild steel surface. Enhancement of inhibition efficiency was observed on account of the concentration of inhibitors. We observed 78% inhibition efficiency for Asparagus racemosus extract at 300 ppm inhibitor concentration, 88% inhibition efficiency for the plant extract of Voila odorata at 300 ppm inhibitor concentration and 89% for Tinospora Cardifolia at 120 ppm inhibitor concentration with 8% H2SO4. Therefore, it is clear that Tinospora Cardifolia extract shows maximum inhibition efficiency of 89% with 120 ppm inhibitor concentration and 8% H2SO4, hence it can be a better corrosion inhibitor. Present study may be useful for all those areas where mild steel meets acidic medium or wherever mild steel corrosion occurs
Authors gratefully acknowledge Dr. R. Jayaganthan, Department of Metallurgical and Materials Engineering, IIT Roorkee, Uttrakhand, India for provide lab facility for our study.