ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Velmurugan.K1 and Sathiyagnanam A.P2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Scarcity of conventional petroleum fuels has promoted research in alternative fuels for internal combustion engines. Worldwide energy demand has been growing steadily during the past five decades and most experts believe this trend will continue to rise. The conventional petroleum fuels for internal combustion engines will be available for few years only, due to a tremendous increase in population. Biodiesel is a green fuel produced from renewable sources, offers cleaner combustion over conventional diesel fuel. However, several authors report to increase in NOx emissions (about 13%) for biodiesel compared with conventional diesel fuel. In this paper, the effect of antioxidant additive L-ascorbic acid on NOx emissions in a Mango Seed methyl ester fuelled direct injection diesel engine have been examined experimentally and compared. The antioxidant additive is mixed in various proportions (100-400mg) with Mango Seed methyl ester is tested on a kirloskar make 4-stroke water cooled single cylinder diesel engine of 5.2 KW rated power. Results show that the antioxidant additive is effective in controlling the NOx and HC emissions of Mango Seed methyl ester fuelled diesel engines.
Keywords |
| Biodiesel, mango seed oil, antioxidants, L-ascorbic acid, fuel additives, engine emissions |
INTRODUCTION |
| Biodiesel refers to mono-alkyl esters of long-chain fatty acids prepared from plant oils, animal fats (or) other lipids. The production of biodiesel has been growing worldwide and will continue to do so because of the rising price of petro diesel [1,2]. NOx is a major cause of Smog, ground level ozone also a cause of acid rain. The fuel NOx is considered as negligible, it does not contain fuel bound nitrogen. Among the all radical formations during combustion, hydroperoxyl (-OOH), hydroxyl (HO-), alkoxyl (RO-) and peroxyl (ROO-) radicals have significant impact on NOx formation. These radicals react with N2 and N2O to form nitrogen oxides. Furthermore, antioxidants can reduce free radical formation by four routes; chelating the metal catalysts, chain breaking reactions, reducing the concentration of reactive radicals and scavenging the initiating radicals. The objective of this research is to investigate the effect of antioxidants on NOx formation of a mango seed derived biodiesel fuelled direct injection diesel engine [3, 4]. In spite of its lower heating value, biodiesel also has a lower stoichiometric air- fuel ratio. Thermodynamically, adiabatic flame temperature is a function of heating value and stoichiometric air/fuel ratio. Benajes et al [5] show significant effects of adiabatic flame temperature, heat release rate and stoichiometric burning on NOx formation in diesel engines. The adiabatic flame temperature of biodiesel is reported to have slightly higher than petro –diesel due to complete combustion resulting from fuel bound oxygen[6].Szibist et al.[7], however, observe a lower rate of heat release for the biodiesel fuel at full loads. The reduced soot formation of biodiesel leads to decreased radiative heat transfer which results in increased combustion temperature and NOx. Yuan and Hansen [8] conducted computational study using Zeldovich NOx formation model together with a Kelvine Helmholtz Rayleighe Taylor (KH-RT) spray break-up model and conducted that decreased spray cone angle and advanced start of injection of biodiesel influences NOx formation. Biodiesel requires longer pulse-width than diesel in electronic controlled engines due to its lower calorific value causing more quantity of fuel entry into the cylinder which results in high temperature and NOx [9]. Varuvel et al.[10] concluded that the increased premixed combustion of biodiesel fuel is one of the reasons for biodiesel NOx effect. Since biodiesel contains oxygen, it premixes more fully during the ignition delay, and a larger fraction of its heat release occurs during the premixed –burn phase of combustion at ignition. Combustion that is more premixed has higher oxygen concentrations and therefore produces more NOx. Allen and Watts [11] stated that the sauter mean diameter of the methyl ester biodiesel varies from 5 to 40% higher than petroleum diesel fuel. An increase in the sauter mean diameter reduces the premix phase of combustion , causing an increase in the diffusion phase of combustion and NOx. CH and OH radicals are continuously formed during combustion reactions. The formation of CH-radicals is an indicator of low temperature pre- combustion reactions, which is the first step for the combustion process, once fuel is evaporated. OH radicals are formed during high pressure reactions and are located in the flame front, where vaporized fuel reaches the highest temperatures [12]. Brezinsky et al.[13] claimed that the high rate of acetylene production from the unsaturated fatty acids of biodiesel is the major cause of increased NOx formation. The acetylene is , responsible for hydrocarbon CH radical generation and prompt NOx. Many researchers have found that the higher NOx emissions produced in biodiesel combustion is influenced by factors such as the physic chemical properties, molecular structure of the biodiesel, adiabatic flame temperature, injection timing and ignition delay time and so on[14]. Higher biodiesel NOx emissions occur mainly due to the increase in the formation of prompt NOx in biodiesel combustion in diesel engines [15]. NOx is generated during combustion by three main mechanisms: thermal, prompt and fuel. Thermal NOx is generated by oxidation of nitrogen at elevated temperatures (above 1700K), while prompt NOx is generated by the formation of free radicals in the flame front of hydrocarbon flames and it is considered as moderately significant compared to total NOx formation. Thermal and prompt are the dominant mechanisms for the NOx generation during combustion, thermal mechanism is largely unaffected by fuel chemistry, where as the prompt mechanism is sensitive to free radical concentration within the reaction zone. The free radicals formation during combustion determines the rate of reaction and prompt NOx production [16]. Free radical is a highly reactive molecule with one or more unpaired electrons. Antioxidants inhibit oxidative processes by donating an electron or hydrogen atom to a radical derivative. The mixture of antioxidant and biodiesel fuel suppresses the peroxyl free radical formations by reaction with aromatic amines. These peroxyl free radicals are the main cause of the higher biodiesel NOx emission [17]. In this study, Lascorbic acid is chosen as the antioxidant test additive to reduce NOx emissions based on cost, effectiveness and availability. The L-ascorbic acid antioxidant is purchased from Sd-fine chem. Ltd, India. |
 |
 |
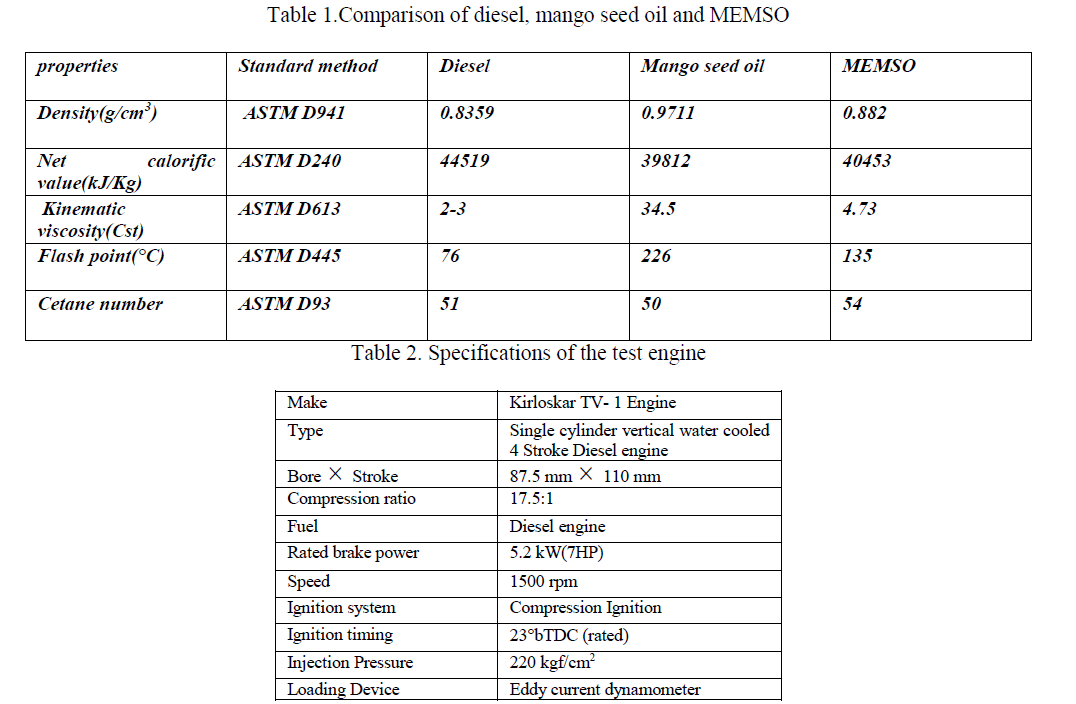
EXPERIMENTAL SET-UP |
| Experiments are carried out in a single-cylinder, water -cooled, naturally aspirated direct injection diesel engine of 5.2 KW rated power coupled with an eddy current dynamometer. An eddy current dynamometer coupled to the engine is used as a loading device. The gas flow rates, fuel flow rate, speed, load and exhaust gas temperature are displayed on a personal computer. Exhaust emissions are measured with a NDIR (Non-Dispersive Infrared) based AVL DiGas 444 gas analyser. The analyser provided a CO measurement range of 0 to 20% by volume with a resolution of 0.01%, NOx range of 0 to 5000 ppm with a resolution of 1 ppm and HC range of 0 to 20,000 ppm with a resolution of 1 ppm. L-ascorbic acid is accurately weighed using a high-precision electronic weighing balance and added to measure quantity of mango seed biodiesel. To make 100 ppm of antioxidant mixture 100mg of the antioxidant is added to 1 kg of the biodiesel. A 3000 rpm speed mixer is used to prepare a homogenous mixture of the antioxidant and fuel. |
RESULTS AND DISCUSSION |
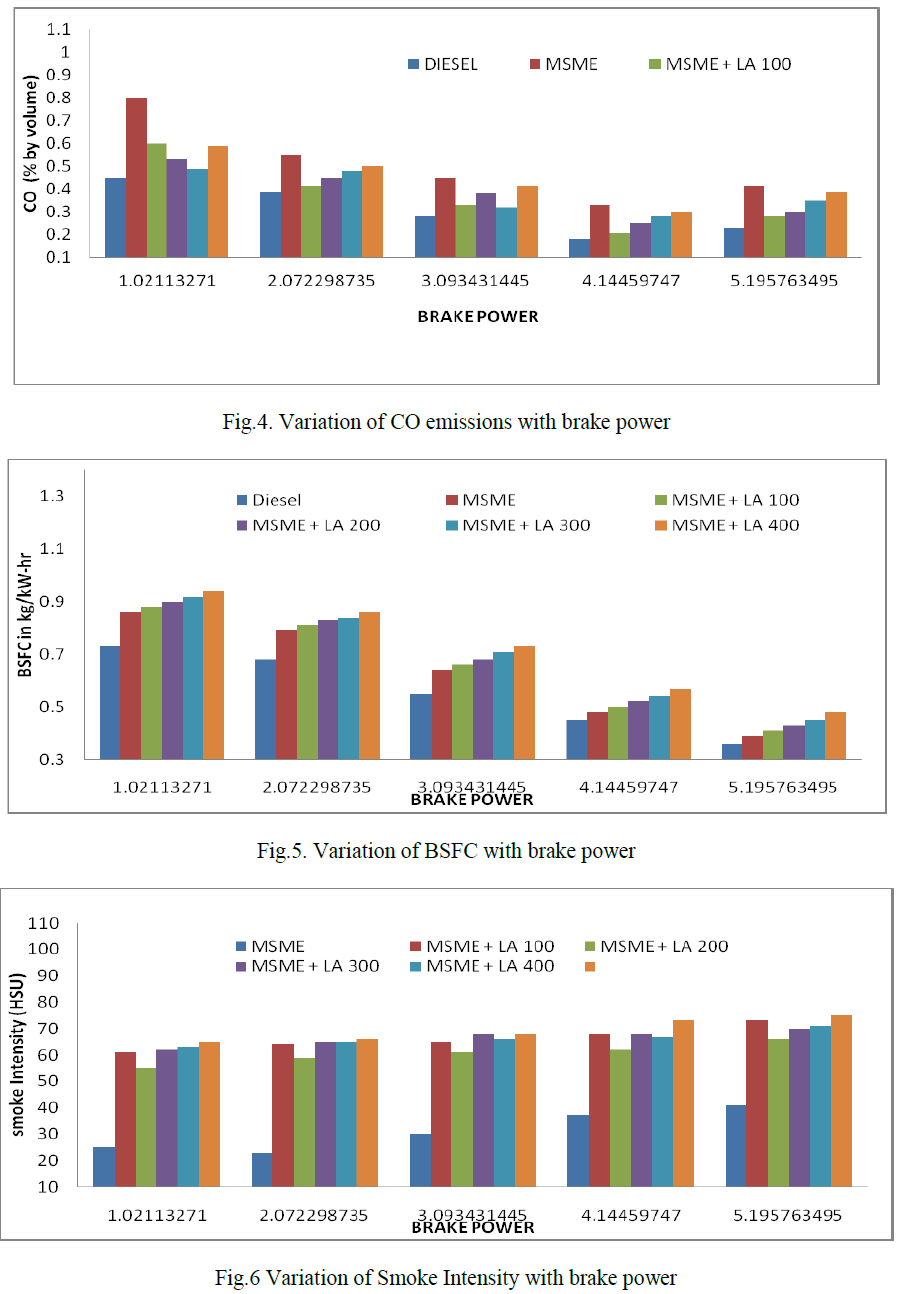
| The effect of antioxidant additives on NOx, HC, CO2, CO and smoke intensity with mango seed methyl ester is investigated in this study. The NOx measurements are repeatable within each engine run, with replicate measurements varying by 3-8 ppm. As per the specifications of the analyser the maximum possible error in the measurement of NOx, CO and HC emissions are ±5%. |
| Comparison of NOx emissions: |
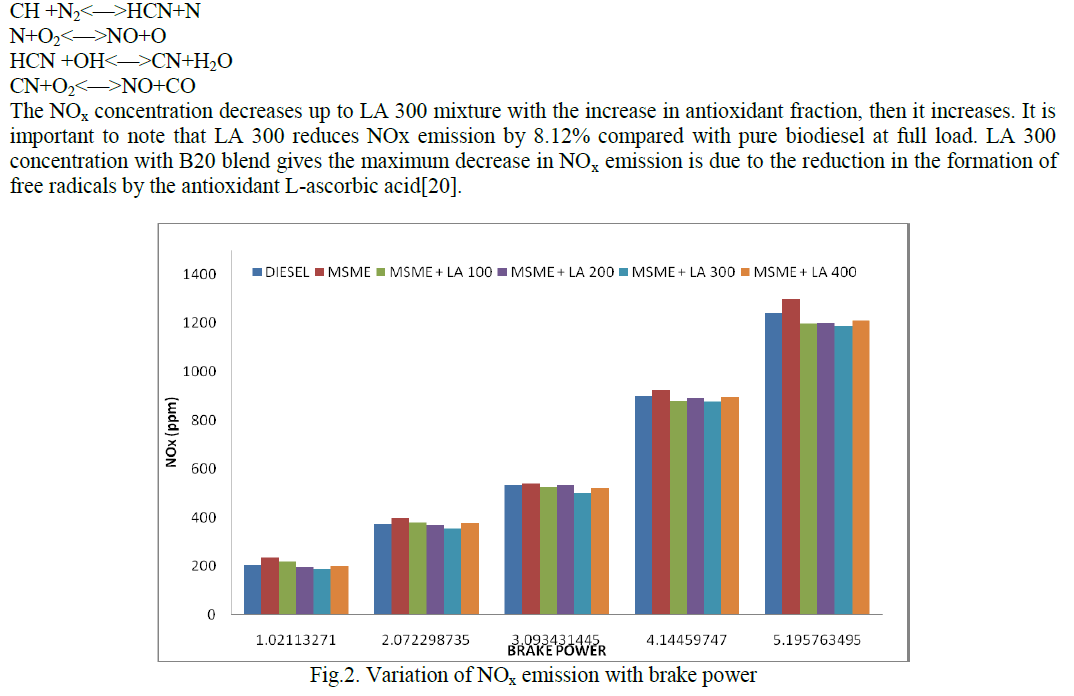
| Fig.2 shows the variation of NOx with brake power. Results indicate that significant reductions in NOx is observed while using antioxidants and the reduction is not linearly correlated with the amount of antioxidants present in the biodiesel. L-ascorbic acid is a radical-trapping antioxidant that scavenges reactive nitrogen oxide species such as NO, NO2 and N2 to prevent nitrosation of target molecules [18]. The main reason for the NO increase with the use of mango seed methyl ester is discussed by Fennimore [19] mechanism and it is proposed that NO formation is initiated by reactions of hydrocarbon radicals (CH,CH2, C2,C and C2H) with molecular nitrogen. The production rates of HCN, N and NO increase with the concentration of CH radicals. |
 |
| Comparison of HC emissions: |
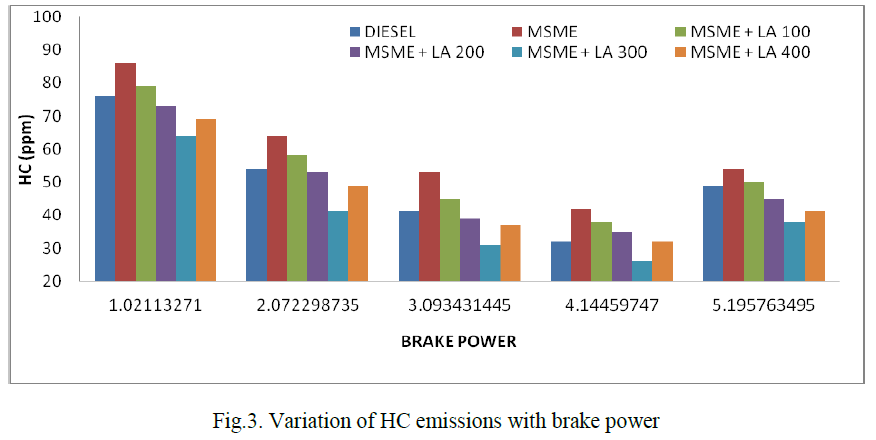
| Fig. indicates the variation of HC emission with brake power. Generally unburned hydrocarbons come under different forms such as drops of fuel, vapour and products of fuel after thermal degradation. The HC emission is found to be lower when the antioxidant traction in the biodiesel is increased. LA 300 concentration with B20 blend reduces the HC emission by 22.8% at full load condition. Generally, all antioxidant mixtures reduce HC compared with pure mango seed methyl ester. L-ascorbic acid reduces the functional groups present in the Mango seed methyl ester and basically it’s a reducing agent. Having high oxygen content in the Mango seed methyl ester leads to prolonging complete combustion and results in lower HC emission. |
| Comparison of CO emissions: |
| Fig. shows the variation of carbon monoxide with brake power. It is observed that biodiesel blends reduce CO emissions significantly compared to diesel. CO emission is formed due to incomplete combustion. Too rich fuel -air ratio and low flame temperature are the major causes of CO emissions from engine. The oxidation of CO is related to the amount of OH radicals present in the reaction. The antioxidant concentration is the Mango seed methyl ester slightly reduces CO emission then it increases. |
 |
| Comparison of brake specific fuel consumption: |
| Fig. shows the variation of brake-specific fuel consumption with brakepower. The specific fuel consumption of the L-ascorbic acid is slightly higher when compared with neat biodiesel , it may be by a little more fuel supplied to the engine to compensate the slight power loss due to the incomplete combustion and improper combustion. |
| Comparison of smoke intensity |
| Fig. shows the variation of smoke intensity with the brake power for diesel and biodiesel-antioxidant mixtures. The results show that smoke intensity increases with the increase in engine load. The smoke intensity is increased at all load conditions when the antioxidant concentration slightly increases and it may be due to improper combustion resulting from antioxidant addition. |
 |
CONCLUSION |
| The effect of antioxidant L-ascorbic acid addition on NOx, HC,CO and smoke intensity with Mango seed methyl ester fuelled DI diesel engine at different concentrations have been reported in this paper. Results show the benefit antioxidant additives for NOx reduction in biodiesel fuelled diesel engines. The main conclusions of the study are: |
| 1. L-ascorbic acid is quite effective in controlling NOx formation. However, it has more CO and HC emissions. Due to the addition of LA 300 with Mango seed methyl ester, the NOx emission is reduced by 21.33% and also HC emission is reduced by 8.12% at full load condition compared with MSME. |
| 2. The remaining mixtures of LA 100, LA 200, LA 400 with Mango seed methyl ester, the NOx emissions are reduced by 4.22%,7.57%,6.94% respectively, and further HC emissions are reduced by 5.32%,11.64%,17.23% respectively at full load condition compared to pure biodiesel. |
| 3. Slight increase in CO emission, BSFC is observed with L-ascorbic acid compared to mango seed methyl ester. Smoke intensity for all mixtures found to be increased slightly due to the disturbance during combustion. |
References |
|