ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| R.Sonia1 and R.Ramanibai2* |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Cyanobacterial blooms are the most common problem in eutrophic or hyper eutrophic water bodies. The blue green algae, Microcystis aeruginosa occurred and dominated during the summer season in Velacherry Lake. Along with M. aeruginosa, M. flos-aquae and M. wesenbergii co inhabit the freshwater habitats. Molly fish were used as animal models to detect the toxicity of microcystins consumed along with the diet intake. Fish were exposed to a known quantity of Microcystis (6 X 104 cells/mL) mixed in an aquarium tank. Survival rate and growth rates were monitored daily. Soon after death, various vital organs of the experimental fish were removed for histopathological analyses. Results revealed that the mortality of the Molly fish may have occurred due to the toxic effect of microcystins. Signs of toxicity exhibited by Molly fish indicated that the bloom samples were predominantly hepatotoxic in nature.
Keywords |
| Poecilia, Microcystis, microcystins, toxicity, cyanobacteria. |
INTRODUCTION |
| Cyanobacteria are prokaryotes that possess cell walls composed of peptidoglycon and lipopolysaccharide layers inside the cellulose of green algae. Morphological diversity of cyanobacteria ranged from unicellular levels, to small colonies of cells, to simple and branched filamentous forms [9].Toxic blooms of cyanobacteria has lead to death of livestock and has caused illness and sometimes even death in human beings. [3, 7]. |
| Cyanobacteria have a number of special properties besides their ability to fix N2 using the enzyme nitrogenase. In general, the nutrient overload favours the cyanobacterial bloom development. The freshwater cyanobacteria, such as Microcystis, Anabaena, Oscillatoria and Nostoc have been reported to produce toxins known as hepatotoxic microcystins[6]. |
| Microcystin have also accumulated in aquatic vertebrates [14,19]. Mohammed Hussain [18] studied the accumulation of microcystins present in different vital organs particularly in the liver of fish. |
| A brief experimental study was carried out to evaluate the toxic effects of microcystins produced by cyanobacteria on Molly fish (Poecilia sphenops) through histopathological examinations. |
MATERIALS AND METHODS |
Study Area |
| Velacherry Lake, located in the southern part of Chennai city, is an example of an aquatic ecosystem suffering from various kinds of environmental impacts. This lake is situated at 12º 59’ 15” latitude and 80 º 30’ 45” longitude with a capacity of 63 mc ft covering an area of about 962 acres and its full tank level is 6.550 m. It is utilised by the public for various purposes other than drinking. |
Microcystis Collection |
| The sampling was conducted during the high algal bloom from the Velacherry lake using 50 μm plankton net. From the algal samples the toxic cyanobacteria were identified and isolated (Table1). |
 |
Poecilia sphenops Capture and Maintenance |
| Healthy fish with a 5±1 cm length devoid of cyanobacteria as well as other parasitic infections were purchased from a nearby aquarium shop. The fish tanks were set up with continuous aeration under room temperature. The fish were fed with commercial fish food and were acclimatized for one week prior to the start of the experiments. |
Experimental Design |
| One control and two experimental fish in each tank were maintained for the experiment. The cyanobacteria Microcystis aeruginosa, M. flos-aquae and M. wesenbergii were given as the diet at a concentration of 6 X 104 cells/mL to each fish individually. Survival rate was monitored and body weight of the fish measured daily. Soon after mortality, the fish were sacrificed and the tissues (liver, gills and ovary) were fixed in aqueous Bovines fluid before histochemical analyses. Finally, 5μ sections were stained with Haematoxyllin and observed under the microscope for histopathological changes. Serum bilirubin was estimated in the liver by the method of Malloy and Evelyn [17]. |
RESULTS |
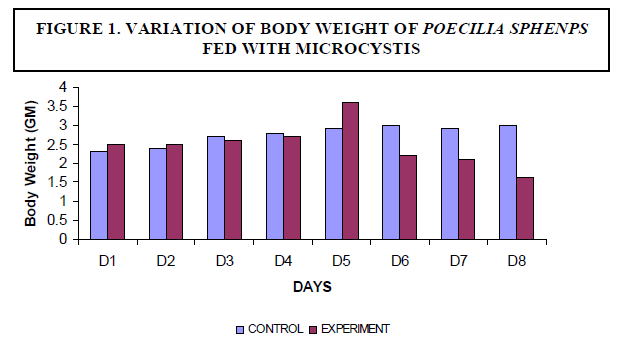
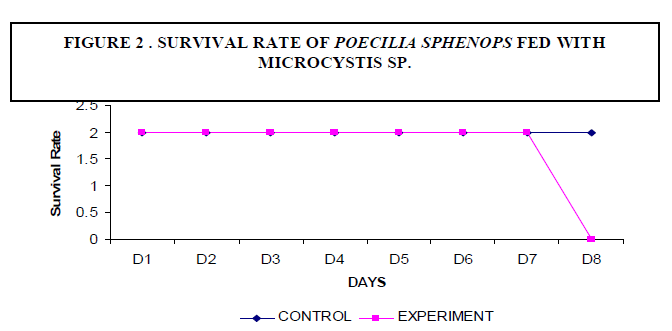
| A decrease in the body weight of the experimental fish was observed. Body weight of the fish measured 2.3±2 gm initially and decreased to a final weight of 1.6±5 gm (Figure1). There was no mortality of fish noticed among the control fish. The experimental fish survived up to seven days. On the eighth day fish mortality occurred (Figure 2). |
 |
 |
Bilirubin Estimation |
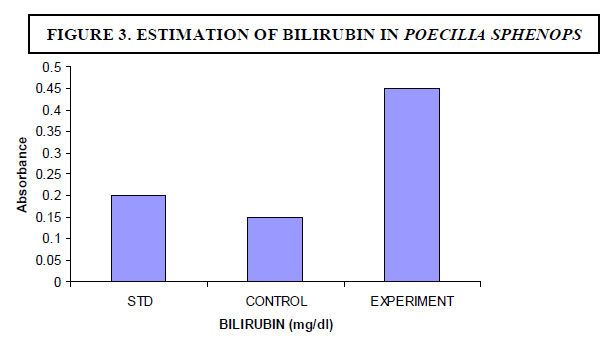
| The experimental fish were fed with Microcystis sp. The concentration of bilirubin was 0.1 mg/dl for control fish whereas the experimental fish showed 0.45 mg/dl (Figure 3). The breakdown of heme in red blood cells results in the formation of bilirubin - the main bile pigment. Hepatocyte necrosis occurred when Microcystis was given as a diet. |
 |
Histopathological studies |
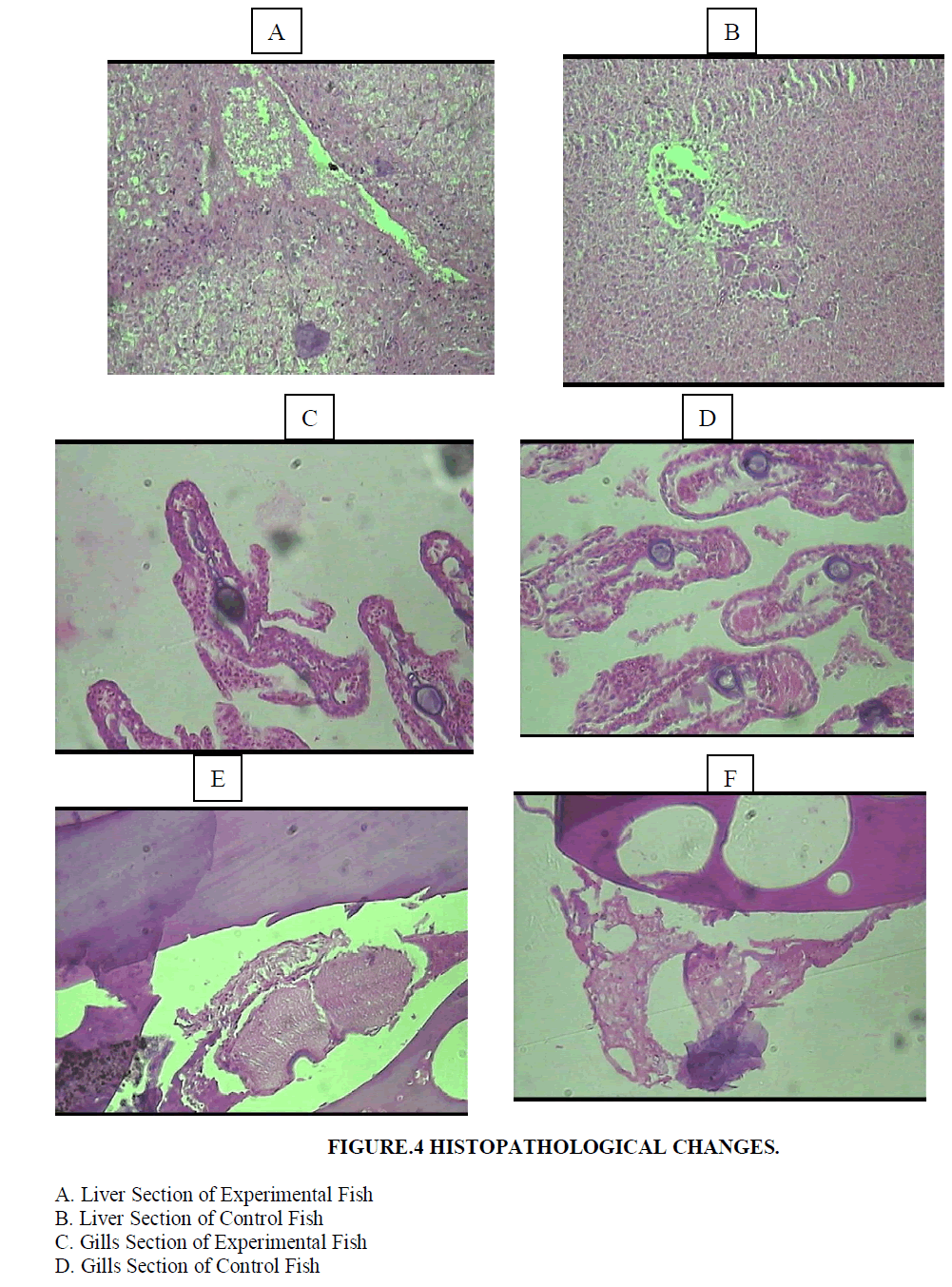
| Histopathological examination carried out on the liver of the experimental fish (Poecilia sphenops) showed mainly architectural changes in the liver (Figures 4A and 4B). Parenchyma architecture of the liver was disturbed and hepatocytes appeared to be swollen with granular cytoplasm. The hepatocyte cells showed condensed chromatin material with sinusoidal epithelium. Blood spilling into the liver tissues and hepatocyte necrosis was observed. Normal nucleus and hepatocytes were seen in the control fish tissues. |
| Control fish exhibited straight gill lamellae with compact cellular structure and large numbers of mucous cells at the tip of primary lamellae. Experimental fish showed hypertrophy in epithelial cells, lamellar aneurism and vascular congestion. The mucous cells were large and prominent (Figures 4C and 4D). Necrosis, epithelial lifting and lamellar fusion, lamellar aneurism, hypertrophy, apoptosis in the cells, and lamellar blood dilation were observed in the gills. |
| Control fish showed mature oocytes with nucleus and nucleolus and oocytes filled with yolk granules. Experimental animals showed ruptured follicle walls in the oocyte and the compactness of the tissue was also disturbed. The developing oocyte had less yolk, follicle cells were deformed and necrosis was also observed at some places in the ovary (Figures 4E and 4F). |
 |
| E. Ovary Section of Experimental Fish |
| F. Ovary Section of Control Fish |
DISCUSSION |
| Mohammed and Hussein [18] reported an accumulation of microcystins in different organs, particularly in the liver of tilapia fish (Oreochromis niloticus) from Egyptian fish farms with toxic blooms of Microcystis aeruginosa. Microcystins also serve as tumour promoters. Microcystis blooms occur in quiet warm water with nutrient rich conditions. Serious illnesses such as hepatoenteritis, symptomatic pneumonia and dermatitis may result if humans consume or have contact with water contaminated by the toxin producing cyanobacteria [10, 27]. The broad specificity anion transport bile acid carrier plays a main role in the entry of toxins into hepatocytes of the liver and other targeted organs [24]. Microcystins are very stable in water and show resistance to pH extremes and temperatures up to 300ºC. |
| The impact of cyanobacteria on fish populations is not only due to the toxins that they release but they also create anoxic conditions and release of compounds during and after bloom collapse [2,1]. Nevertheless, Shebulsky [25] proved that fish death occurred in the presence of a toxic strain of M. aeruginosa with and without an oxygen supply indicating that toxins were also involved in the fish kill. |
| Tancella et al., [27] proved that microcystin administered orally to fish caused massive hepatic necrosis followed by death. The same results were also observed in this study. Obrehm et al., [20] reported that microcystin may affect the development of fish embryos. Cyanotoxins are usually found inside the cells being degraded very slowly [21,26,23]. Animal intoxication may occur either by direct ingestion of liver cells or by drinking contaminated water after the bloom collapses and toxins are released from the cells. |
| The liver was the most affected organ in all such cases because microystins were found to be potent hepatotoxins for mammals and fish [8,11]. Microcystins accumulate mainly in the liver but also in other organs, for example, the kidneys, gills and intestine[22,30,13,16]. In the present study, the accumulation of microcystins was observed in the liver, gills and gonads. |
| Cabris et al., [5] reported an increase in the level of bilirubin concentration in Cyprinus carpio due to microcystin intoxication. The same results were obtained in the Molly fish (Poecilia sphenops). In the present study, apoptotic cells were formed in the liver, gills and ovary of the experimental fish. |
CONCLUSION |
| Oral doses of cyanobacteria can cause enhanced physiological stress as well as continued pathological changes in the liver, gills and ovaries possibly leading to decreased functionality of these organs. The combination of stress and organ damage might explain the reduced weight and mortality of the fish. |
Acknowledgement |
| One of the authors (R.R) thank UGC New Delhi for funding (MRP) support and R. Bharathi for the laboratory assistance. |
References |
|