e-ISSN: 2319-9849
e-ISSN: 2319-9849
Divyamshu Surabhi1* and Dr. Nagamanju S2
1Department of Biotechnology, Chaitanya Bharati Institute of Technology (CBIT), Hyderabad, Telangana, India
2Department of Genetics and Biotechnology, Bhavan’s Vivekananda College of Science, Humanities and Commerce, Hyderabad, Telangana, India
Received Date: 31/08/2021; Accepted Date: 13/10/2021; Published Date: 20/10/2021
Visit for more related articles at Research & Reviews: Journal of Chemistry
Energy is one of the fundamental units that drive various processes. Among them, heat or thermal energy is quite abundant in nature; usually released outside the system as a byproduct. The total summation of internal energy stored within reactants or products is Enthalpy (H). The amount of heat lost or gained by a system is equal to the Change in enthalpy of combustion or ΔH. In the present study an attempt has been made to investigate whether the positioning of OH functional group in methanol, 2-propanol and 2-methylpropan-2-ol has any effect upon the standard enthalpy change. Calorimeter was used to calculate the standard change in enthalpy of combustion of alcohol, by raising the temperature of 100 g of distilled water by 30oC from its initial temperature. The water was heated using a spirit lamp with 20 ml of specific alcohol; it’s mass before and after combustion was recorded. Procedure was repeated three times with all three alcohols. The final values obtained were -41.8 ± 0.10 KJ mol-1, -114 ± 0.39 KJ mol-1, -114 ± 0.38KJ mol-1 for methanol, propan-2-ol and 2-mehtylpropan-2-ol respectively. The present study showed that standard enthalpy changes of combustion increased from primary to secondary alcohol. This is due to the same number of C-C bonds or same carbon chain length. Only a slight variation of ± 0.01 KJ mol-1 was observed. This variation exists as the standard enthalpy change of combustion is not only dependent on the nature of the C-OH bond but also neighboring groups as they have different enthalpy of formation. To conclude the standard change in enthalpy of combustion of alcohols is affected by the number of C-C bonds, its R group and by the phenomenon of geometrical isomerism.
Enthalpy, Thermochemistry, Heat capacity.
Energy is one of the fundamental and most fascinating units that make up the fabric of reality. Without energy nothing would exist! As it is the driving force behind the actions and reactions of all things. Talking about driving force; one would come across two terms; Work and Heat. Work is defined as the energy transferred to the motion of objects. Heat is the energy transferred to the motion of atoms and molecules [1]. All things in existence tend to do some work during at some point and mostly encounter one of the common forms of energy called heat or thermal energy. Especially if the work is done on earth. Since, heat is so common and partially omnipresent; hence present study intends to explore the realm of thermodynamics or to be specific thermochemistry.
Thermochemistry refers to the study of heat energy absorbed or released during a chemical reaction. These reactions can either be exothermic: release of heat energy or endothermic: absorption of heat energy [2]. The energy captivated within the system is represented as enthalpy. Enthalpy (H) is defined as the sum of the total internal energy stored in the reactants or products. However, one cannot determine absolute enthalpy due to numerous un-controlled variables involved, but the change in enthalpy can be calculated. Change in enthalpy of combustion or ΔH is equal to the amount of heat lost or gained by the system. It is calculated under the assumption that the pressure acting on the system is constant. Thus, the equation ΔH = mcΔt or q (notation for the energy gained or lost by the system) arises [3].
Combustion is defined as an exothermic reaction that occurs between a fuel and an oxidizing agent eventually releasing
energy in the form of heat and light. The present study focuses on the standard enthalpy change of combustion 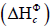 . It is when one mole of a substance undergoes complete combustion in presence of oxygen under standard conditions (298 K and 100 KPa
pressure) [4].
. It is when one mole of a substance undergoes complete combustion in presence of oxygen under standard conditions (298 K and 100 KPa
pressure) [4].
Based on the studies on the correlation between the length of carbon-chain and the enthalpy of combustion of 5 primary alcohols [5]. Henceforth, present research aims to find “How would the position of the OH functional group in alcohols effect the standard enthalpy change of combustion?” As the position of OH functional group effects the physical and chemical properties of an alcohol; the change in such properties might have an impact upon the standard change in enthalpy of combustion of alcohols.
Alcohols are hydrocarbon chains that contain a hydroxyl (-OH) functional group with a general formula of Cn H2n+1 OH. Alcohols are classified into three categories: primary, secondary and tertiary [6].
Primary alcohols
Alcohols that are classified into this category have their carbon atom of hydroxyl group (-OH) bonded to a single alkyl group. Possible examples are ethanol and methanol (Figures 1 and 2).
Secondary alcohols
Here the carbon atom of the hydroxyl group (-OH) is bonded to two alkyl groups on either side. Examples are propan- 2-ol and butan-2-ol (Figures 3 and 4).
Tertiary alcohols
Have their carbon atom of the hydroxyl group (-OH) bonded to 3 alkyl groups. For example, 2-methylpropan-2-ol and 2-methylbutan-2-ol (Figures 5, 6 and Table 1).
| Variables | |
|---|---|
| Independent | The type of alcohol used (primary, secondary, tertiary) |
| Dependent | The mass of the alcohol burned/combusted |
| Controlled | |
|
|
Table 1A. Variables.
| Apparatus | Materials |
|---|---|
| Weighing balance (± 0.01) | Methanol (Primary alcohol) |
| Thermometer (± 0.5) | Propan-2-ol or Isopropyl alcohol (Secondary alcohol) |
| Copper calorimeter | 2-methylpropan-2-ol (Tertiary alcohol) |
| 3 different spirit lamps to prevent cross contamination. | Distilled water |
| Wind shields | Tissues |
| Burette stand | -- |
Table 1B. Apparatus and materials.
To examine the effects of the positioning of the OH functional group. The following alcohols were experimented.
• Methanol (Primary)
• Isopropyl alcohol or 2-propanol (Secondary)
• 2-methylpropan-2-ol (Tertiary)
• Supervision is recommended when experimenting with highly combustible liquids and vapours.
• Usage of gloves to avoid skin contact as it may cause moderate skin irritation and accidental ingestion may lead to gastrointestinal irritation.
• Always wear safety goggles as any contact with the eye may cause severe eye irritation.
Experimenter is suggested to wear a mask while conducting their experiments as inhalation in excess may cause nausea, headache resulting in unconsciousness.
Experimental set up
Primarily, the standard copper calorimeter was insulated by wrapping a thin layer of sponge and around with a layer of aluminium foil; to prevent the sponge from melting.
In a similar fashion, a lid was made to prevent heat loss. Through which a thermometer was placed and clamped to a burette stand; to prevent the thermometer tip from touching the copper bottom– which could alter the accuracy of the temperature readings.
Later the insulated calorimeter was placed on a tripod stand under which the spirit lamp was placed. The spirit lamp was slightly elevated with the help of a small box. The same small box was used throughout the experiment to maintain the distance between the spirit lamp and the calorimeter constant. Three wind shields (wooden boards) were placed all around the system to shield the flame from wind gusts as shown in Figure 7.
1. Measured 100 ml or 100 g of distilled water and poured into the calorimeter.
2. Recorded the initial temperature of the water
3. Measured 20 ml of the alcohol and weighed its mass. And poured the alcohol into the spirit lamp.
4. Stationed the wind shields around the system to prevent heat loss and to protect the flame from wind gusts.
5. Spirit lamp was lit and immediately placed under the calorimeter.
6. Experiment continued until the temperature of the water increased by 30℃ form its initial temperature.
7. Flame was put out and immediately after a 30℃-rise in temperature from initial.
8. And the mass of the alcohol was measured immediately i.e., to prevent the alcohol vapours from escaping as alcohols are highly volatile in nature.
9. Spirit lamp was let to cool down for a minute. Meanwhile, the left-over alcohol was poured into a beaker and placed away from the experimental zone to prevent any fire accidents.
10. Previous steps were repeated for all the 3 alcohols with minimum of 3 trials each.
Note: It is highly recommended to use different spirit lamps for each alcohol to prevent cross contamination.
Data analysis
Due to the spirit lamp being over 200 g (above the capacity of the sensitive balance available). A beaker of 61.05 g was used to weigh the mass of the alcohol throughout the investigation (Tables 2-4).
| Data collection (Methanol) | ||||
|---|---|---|---|---|
| Beaker’s weight = 61.05 grams (±0.01) Water taken = 100 grams or 100 ml (±0.01) |
||||
| Methanol | Initial mass (g) ± 0.01 |
Final mass (g) ± 0.01 |
Initial Temp (°C) ± 0.5°C |
Final Temp (°C) ± 0.5°C |
| Trial 1 | ||||
| 32.08 g | 20.53 g | 25°C | 55°C | |
| Trial 2 | ||||
| 30.79 g | 22.17 g | 25°C | 55°C | |
| Trial 3 | ||||
| 33.54 g | 24.79 g | 24°C | 54°C | |
Table 2. Raw data – Methanol.
| Data collection (Propan-2-ol) | ||||
|---|---|---|---|---|
| Beaker’s weight = 61.05 grams (±0.01) Water taken = 100 grams or 100 ml (±0.01) |
||||
| Propan-2-ol | Initial mass (g) ± 0.01 |
Final mass (g) ±0.01 |
Initial Temp (°C) ±0.5°C |
Final Temp (°C) ±0.5°C |
| Trial 1 | ||||
| 33.63 g | 25.49 g | 23°C | 53°C | |
| Trial 2 | ||||
| 32.46 g | 28.16 g | 24°C | 54°C | |
| Trial 3 | ||||
| 32.44 g | 24.46 g | 24°C | 54°C | |
Table 3. Raw data - Propan-2-ol.
| Data collection (2-methylpropan-2-ol) | ||||
|---|---|---|---|---|
| Beaker’s weight=61.05 grams (± 0.01) Water taken=100 grams or 100 ml (±0.01) |
||||
| 2-methylpropan-2-ol | Initial mass (g) ±0.01 |
Final mass (g) ±0.01 |
Initial Temp (°C ) ±0.5°C |
Final Temp (°C ) ±0.5°C |
| Trial 1 | ||||
| 32.46 g | 25.41 g | 24°C | 54°C | |
| Trial 2 | ||||
| 31.46 g | 24.46 g | 24°C | 54°C | |
| Trial 3 | ||||
| 31.43 g | 24.39 g | 25°C | 55°C | |
Table 4. Raw data - 2-methylpropan-2-ol.
Data processing
The above calculated data was used to calculate the standard enthalpy change of combustion. Under the assumption that all the energy generated or released during the combustion of the alcohol has successfully transferred into the water without any heat loss. The above premise is based on the 1st law of thermodynamics known as law of conservation of energy. It states that the total energy in an isolated system is always constant and that energy can neither be destroyed or created but can only be transferred from one form to another or from one system to another system [9].
Correlating the above law of conservation with Hess’s law; the standard change in enthalpy of combustion was calculated. Hess’s law states that the enthalpy change of a chemical reaction is pathway independent meaning that even if the chemical reaction occurs in one step or a series of steps the enthalpy change of reaction remains the same. Considering that the reaction starts with same reactants and ends with the same products without any external interference [10].
Change in heat energy of the chemical reaction = Change in heat energy of water
∴ ΔHϕ=mcΔt (mass, specific heat capacity, temperature of water) [3]
The above equation satisfies for one mole of the substance undergoing combustion.
Model calculation of change in enthalpy of combustion of methanol
Change in mass of methanol (Trial 1): 32.08 - 20.53 = 11.55 g
Change in mass of methanol (Trial 2): 30.79 - 22.17 = 8.62 g
Change in mass of methanol (Trial 3): 33.54 - 24.46 = 9.08 g
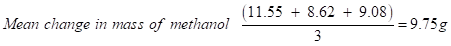
→Mean mass was calculated to reduce the effect of random errors.
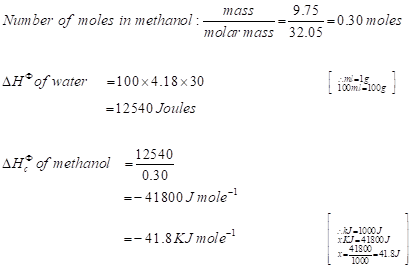
→Negative sign represents the loss of heat energy or the release of heat energy.
The same model calculation will be applied to all the alcohols to calculate their change in enthalpy of combustion (Table 5).
| Trial(s) | Change in mass (g) ±0.02 |
Mean mass (g)±0.02 | No.of moles | ΔHφ of water (J) | ΔHφcof alcohol(s) (J mol-1) |
ΔHφc of alcohol(s) (kJ mol-1) |
|---|---|---|---|---|---|---|
| Methanol | ||||||
| 1 | 11.55 | 9.75 | 0.30 | 12540 | - 41800 | - 41.8 |
| 2 | 8.62 | |||||
| 3 | 9.08 | |||||
| Propan-2-ol | ||||||
| 1 | 8.14 | 6.81 | 0.11 | 12540 | - 114000 | - 114 |
| 2 | 4.3 | |||||
| 3 | 7.98 | |||||
| 2-methylpropan-2-ol | ||||||
| 1 | 7.05 | 7.03 | 0.11 | 12540 | - 114000 | - 114 |
| 2 | 7.00 | |||||
| 3 | 7.04 | |||||
Table 5. Calculating the change in Enthalpy of combustion.
To better represent the obtained results, the absolute uncertainty must be calculated for each alcohol. To calculate absolute uncertainty, the total percentage uncertainty shall be calculated initially. To do so all the uncertainty values of the instruments used in this experiment need to be summarized as per the below method and equation [5].
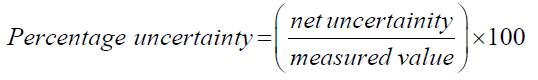
Total percentage uncertainty = % uncertainty of mass of water + % uncertainty of temperature + % uncertainty of change in mass of alcohols.
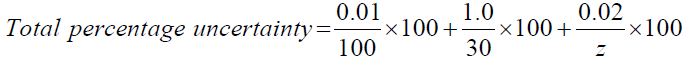
→The variable (z) account for the change in mass of alcohols; since it’s not constant. (Table 6)
| Alcohols | Mean mass of alcohols (g) ±0.02 |
% Uncertainty of alcohol combusted | Total % uncertainty of alcohol combusted |
|---|---|---|---|
| Methanol | 9.75 | ±0.21 | ±0.25 |
| Propan-2-ol | 6.81 | ± 0.29 | ±0.34 |
| 2-methylpropan-2-ol | 7.03 | ±0.28 | ±0.33 |
Table 6. Calculating total percentage uncertainty.
Now, using the above calculated total percentage uncertainty; the absolute uncertainty can be calculated. The absolute uncertainty is the absolute error region under which the desired value can exist. It can be calculated by multiplying the total percentage uncertainty with the measured value.
Model calculation for Propan-2-ol,
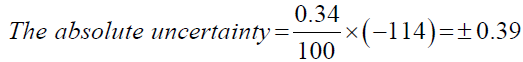
From the above Graph 1, it can be interpreted that the change in enthalpy of combustion alcohols has increased along with the increase in the length of the carbon chain. The obtained values of standard enthalpy of combustion of alcohols are different from the actual values recorded by the scientific community. As they were obtained with the use of 1 mol of alcohol undergoing complete combustion at standard conditions, thus not compared (Table 7).
| Absolute uncertainty | |
|---|---|
| Methanol | ±0.10 |
| Propan-2-ol | ±0.39 |
| 2-methylpropan-2-ol | ±0.38 |
Table 7. Calculating absolute uncertainty.
Current investigation on finding out ‘How would the position of the OH functional group in alcohols effect the standard enthalpy change of combustion?’. The obtained experimental results showcased that the position of OH functional group primary, secondary and tertiary carbon impacts the standard enthalpy change of combustion as it moves from primary alcohols to tertiary alcohols, as displayed in Graph 1. Even-though, the experimental results display that as the position of OH functional group changes, the standard enthalpy change of combustion of secondary alcohol (Propan-2-ol) and the standard enthalpy change of combustion of tertiary alcohol (2-methylpropan-2-ol) exhibited the same amount (-114 KJ mol-1) with a minor difference of ± 0.01 KJ mol-1. The standard enthalpy change of combustion of alcohols is directly affected the length of the carbon chain involved in the reaction [11]. In other words, the enthalpy of combustion of alcohols is highly influenced by the number of C-C bonds involved in the reaction – which implies that more C-C bonds result in a longer hydrocarbon chain and thus more amount of energy is captivated within the covalent bonds. Resulting in higher standard enthalpy of combustion [11].
However, compounds with same number of C-C bonds or same length of carbon chain but different geometrical structures have varied standard enthalpy of combustion values. For example [12]
• 1-butanol: −2671 KJ mol-1
• 2-butanol: −2661.1 KJ mol-1
• Isobutanol: −2662.6 KJ mol-1
• Tert-butyl alcohol: −2644.8 KJ mol-1
This variation tends to exist as the enthalpy of combustion is not only dependent on the nature of the C-OH bond but also the R group. As its neighboring groups have different enthalpy of formation thus, may result in slight variations in enthalpy of combustion. And it also states that if the neighboring R groups of the C-OH bond are same in two different hydrocarbon chains but have different positioning; they still showcase the same enthalpy of combustion. This phenomenon is seen when comparing two hydrocarbon chains that mirror each other known as enantiomers [12].
In conclusion, the present investigation states that the standard enthalpy change of combustion is not solely dependent on the length of hydrocarbon chain but also its geometrical variations and its R group.