ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Jayant Kher, Manisha Khaladkar, Nandini Iyer Assistant Professor in Chemistry, Department of Applied Science, College Of Engineering Pune, Pune, Maharashtra, India |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Thermal decomposition of binary mixtures of La (II) oxide and Cu (II) oxalate in the proportion of 1:1 and 1:3.5 was carried out. The end products after heating of the pure Cu (II) oxalate and the binary mixtures were tested for electrical conductivity from room temperature to 1173K.The conductivity was plotted as variation of resistance „e‟ as a function of reciprocal of absolute temperature. All the pellets showed semiconduction with negative temperature coefficient (NTC) of resistance. The calcination products were characterized by XRD and other techniques.
Keywords |
| CuO, XRD, Cu (II) oxalate, La (II) oxide, NTC, resistivity. |
INTRODUCTION |
| Electrical properties of copper oxide in recent times formed the basis of a large number of experimental investigations owing to the great technical significance of Cu2O and it is easy to prepare copper oxide samples. La2O3 oxide has p-type semiconducting properties. When a binary oxide of Lanthanum (III) is made with CuO in various proportions the conductivity of mixture shows different behavior as compared to pure CuO and La2O3. The resistivity measurements showed that the binary mixture has Negative temperature coefficient of variation in resistance. |
II. EXPERIMENTATION |
| The experimental procedure for these studies is divided as follows. 1. Preparation of Cu(II) Oxalate. Cu(II)Oxalate was prepared by adding the solution of pure K2C2O4 (Potassium Oxalate- A.R grade) drop by drop to a solution of pure Copper Nitrate(A R. grade) 2. Preparation of binary mixtures of La(II)Oxide and Cu(II) Oxalate. Binary mixtures of La(II)Oxide and Cu(II) Oxalate were prepared according to their mole proportion (1:1and1:3.5) by mechanical mixing. 3. Pure Cu(II) Oxalate was heated up to 950o C to decompose it to CuO. 4. Binary mixtures were heated up to 950OC 5. The end products of heating were characterized by XRD (Rikagu Miniflex). 6. The end products after heating of the pure Cu (II) oxalate and the binary mixtures were tested for electrical conduction from room temperature to 900oC.The results are plotted as variation of resistance „eâÃâ¬ÃŸ as a function of reciprocal of absolute temperature. Following table indicates the abbreviations used in the experiment |
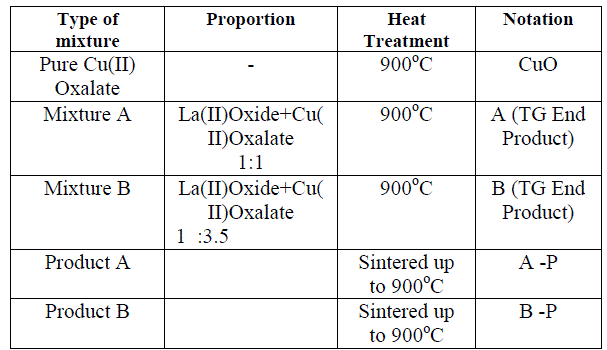 |
| TABLE I Abbreviations Used |
III. RESULTS AND DISCUSSION |
| Electrical Conduction as a function of temperature for CuO, A-P and B –P pellets. Electrical conduction as a function of temperature of CuO, A-P and B-P samples were studied. Results are shown in the Fig. 2 and tabulated in Table 1 and subsequently discussed as follows. XRD of the sintered pellets is shown in (Fig.1a). XRD of the TG end product of mixture A contains La2CuO4 and unreacted CuO and La2O3 phases while the XRD of the sintered pellet A -P (Fig.1b) contains La2CuO4 as a predominant phase. Similarly, TG end product of mixture B contains La2CuO4, and CuO phases while the XRD pattern of the sintered pellet B -P contains La2CuO4 as major phase. Besides the crystalline phases appeared in (Fig.1c) the pellets may be containing non-crystalline phases at the contacts of grain boundaries developed during sintering. |
| As CuO and La2O3 phases are parent oxides in the formation of composite phases, results obtained in the case of composite pellets are compared with those observed in the case of CuO pellet and with those reported for La2O3 pellet. La2O3 in an insulator having band gap ~5 eV[1] and possessing strong dielectric properties[2]. The room temperature conduction of pellet of La2O3 observed using electrometer was found to be ~10-11 ohm-1 therefore; its contribution of conduction carriers to the carriers in composites will not be significant. Apart from this in the present laboratory the preparation of tough pellets of La2O3 was not possible. Hence, all the results obtained in the case of composite pellets are compared with those observed in the case of CuO pellets only. Electrical ConductionFig.2 shows variation of electrical resistance (c) against 1/T, T being absolute temperature of pellets of CuO, A and B samples. All the pellets behave as semiconductors exhibiting negative temperature coefficient of resistance (Table 1). A) Electrical conduction of CuO pellet: Electrical conduction of CuO pellet was studied as follows. In case of CuO pellet the initial resistance (25 k ) decreases gradually with the increase in temperature. From room temperature to 3300C with activation energy E= 2.0 X 10-3 eV. From 375 to 4600C with E, 7.0 X 10 –3 eV and thereafter, the resistance falls rapidly with E= 1.4eV. E Values are calculated according to Arrhenius equation. |
 |
| Earlier investigators have measured the electrical conductivity of sintered CuO pellet and found that below 6500C the oxide is non-stoichiometric, containing excess oxygen and show p-type conduction [3,4] with activation energy of 0.15 eV. The two E values are observed viz; 2.0 x 10–3 and 7.0x10–3 eV in the temperature range 30 to 4600C (Table 1). In their work on measurement on thermo EMF generated in CuO at elevated temperature above 650C Zuev et al. [5] have observed that the phase CuO is unstable at ~ 9000C and local deformations are created during the formation of new phase that is the appearance of unstable stressed Cu2O phase. In the present work two different E values are observed due to formation of Cu2O phase on the grain boundaries of CuO phase after sintering the CuO pellet at 9000C. Initially Cu2O phase remains under stress which is unstable & on cooling the CuO pellet, Cu2O phase gets stabilized on the grain boundaries along with excess oxygen. It appears that the excess oxygen and the newly formed stressed phase Cu2O create two energy levels in the pellet. It was not possible in this work to determine the exact nature of carriers, electrons or holes provided by these two energy levels. In the earlier work intrinsic activation energies 1.4, 1.22 or 1.35 eV are reported [3,6] in the temperature range from ~600 to 8000C while in the present work, E 1.4 eV is observed in the temperature range from 470 to 5300C. |
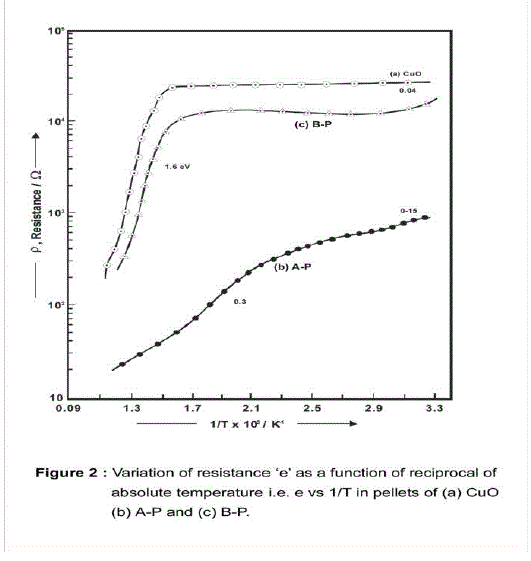 |
| B) Electrical conduction of pellet A-P: In the case of pellets of composites of CuO the variation of resistance is totally different from that observed in the case of CuO pellets. In the case of A pellet the resistance 1.3 K decreases gradually up to 282 in the temperature range from room temperature to 1800C. It decreases up to 800C with E, 0.15eV. If this process continues the resistance will further decrease up to 142 at 1800C, on the contrary the resistance 300 is observed at that temperature. (Fig 2 b) This is possible due to the following processes occurring in that temperature range. The mobility of the conduction carrier in the same applied field decreases, Carriers are supplied by some other phase having positive temperature coefficient of variation of resistance (Metallic behavior) and/or (iii) oxygen on the surface reacts with conduction carriers / electrons and gets chemisorbed over the surface leading to the removal of carriers. In compete pellet the three processes are probable and therefore instead of resistance 142 there is a resistance of 300 at 1800C. |
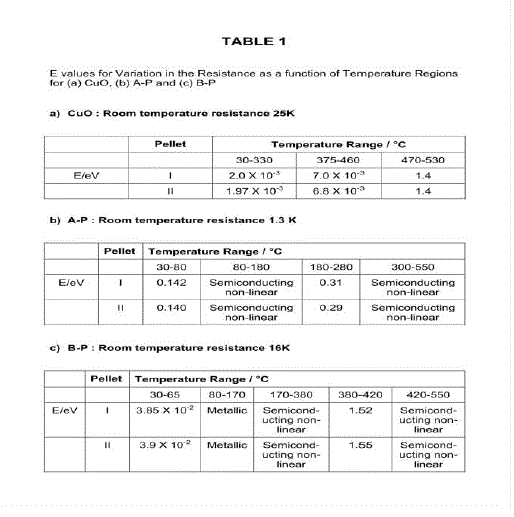 |
| In the temperature range from 180 to 2800C the resistance decreases with E, 0.3 eV and thereafter up to 5500C semiconducting non linear variation is observed between log and 1/T. The non linear variation is also possible due to simultaneous occurrence of two, processes described above. C) Electrical conduction of pellet B-P: In the case of pellet B-P, the variation of resistance shows (Fig 2c) semiconducting behavior as follows: The semi-conducting behavior in the temperature range from 30 to 650C having E= 0.0385 eV, The metallic behavior from 65 to 1700C, Semiconducting non linear variation in log C against 1/T, in temperature range 180-3800C, The semiconducting behavior in the temperature range from 380 to 4200C having E= 1.52 eV and Thereafter up to 5500C semiconducting nonlinear variation of log C against 1/T. The E values are given in Table 1. The metallic behavior and non-linear variation of log observed in the conduction of this pellet can be explained on the basis of the processes described in the case of pellet A-P. Indeed, there exist several phases in both the pellets as observed in their XRD patterns (Fig 1). XRD patterns of pellet A-P shows lines of La2CuO4, Cu2O phases and XRD pattern of pellet B-P displays lines of La2CuO4 and CuO phases. CuLaO2 phase may be present in amorphous state in both the pellets. |
IV. CONCLUSIONS |
| Electrical conduction of CuO changes due to conversion to Cu2O along the grain boundaries of CuO. In the present work two different E values are observed due to formation of Cu2O phase on the grain boundaries of CuO phase after sintering the CuO pellet at 9000C. Initially Cu2O phase remains under stress which is unstable & on cooling the CuO pellet, Cu2O phase gets stabilized on the grain boundaries along with excess oxygen. In case of La2CuO4 (samples A-P and B-P) the conduction depends on carriers. In case of mixture of Oxides after sintering the overall conduction depends upon number of phases formed and their relative proportion. |
References |
|