ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Varsha D Savanth1, Seema J Patel2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Xylanases have huge potential applications in many industries used for the conversion of xylan present in lignocellulose to sugars. Xylanase producing Aspergillus niger islolates were obtained by congo red method of screening from soils collected from different parts of Davangere. Submerged and solid state fermentation were done on natural substrates like- corncob, saw dust and rice husk to check the enhanced production of xylanase. Corncob was found to be the best natural substrate for high activity of xylanase (1.85 units.ml-1) under solid state fermentation compared to submerged fermentation than the standard birch wood xylan substrate (1.22 units.ml-1). The screened, unbleached pulp from sugarcane bagasse was treated with partially purified xylanase and its effectiveness in biobleaching was determined by the Kappa number. The kappa number obtained for enzyme treated pulp showed twenty point reduction than untreated pulp. The %lignin content was also less (two point reduction) for enzyme treated pulp.
Keywords |
| Aspergillus niger, Corn cob, Kappa number, Congo red, Solid state fermentation |
INTRODUCTION |
| Xylan is a heteropolymer and it is a second most abundant biopolymer after the cellulose and the major hemcellulosic polysaccharide found in the plant cell wall [17]. Xylanase is the name given to a class of enzymes which degrade the linear polysaccharide β-1,4-xylan into xylose, thus breaking down hemicellulose [8].Xylanase has aroused great interest recently due to its biotechnological potential in many industrial processes, for example, in xylitol and ethanol production, in the cellulose and paper industry, in the production of oligosaccharides, to obtain cellular proteins, liquid fuels and other chemical substances, in the food industry and in poultry, pork and caprine feeding [6]. |
| The cost of carbon source plays another major role in the economics of xylanase production. Hence, an approach to reduce the cost of xylanase production is the use of lignocellulosic materials as substrates rather than opting for the expensive pure xylans [15]. |
| Filamentous fungi which are main producers of this enzyme from an industrial point of view due to extracellular release of xylanases, higher yield compared to bacteria and yeast and production of several auxiliary enzymes that are necessary for debranching of the substituted xylanase [18]. |
| The interest in cellulase free xylanases has focused on pulping and bleaching processes, in which chlorine (Cl2) and hypochlorine (ClO2) for biobleaching can be reduced. Naturally occurring microbial strains capable of secreting xylanases free of cellulase activity would be attractive for such applications. Xylanases enhance the cleaving of reprecipatated xylan formed on the outer surfaces of the cellulose fibers after pulping [19]. This causes increased permeability of the pulp fibers to the bleaching chemicals and allows the passage of larger fragments of residual lignin out of the pulp. |
II. SCOPE OF RESEARCH |
| Due to their huge potential, xylanases with novel properties from filamentous fungi must be produced, isolated and identified. This study highlights the isolation of xylanases produced by fungal isolates from different soil samples around Davangere. The use of natural substrates for xylanase production can be cost effective and ecofriendly processes. |
III. METHODOLOGY |
Collection of samples |
| The soil was collected from five places in and around Davangere, mainly- soil from rice mill, soil under decaying wood, soil near sugar industry, soil dumped with agro waste and soil from saw mill. From these soil samples, xylanase producing fungi were isolated. |
Primary screening for isolation of fungi |
| Preliminary screening was done by the method of serial dilution technique on media with xylan as carbon source [10]. Isolates of Aspergillus niger were obtained. |
Secondary screening |
| The fungal isolates were screened for their abilities to produce extracellular xylanase during their growth on Czapek’s agar medium containing xylan as the sole carbon source [11]. The inoculated plates were incubated for 7 days at 30ºC. Then the plates were flooded with 0.1% (w/v) Congo red. After 30 min of incubation, plates were washed with 1 M NaCl. The fungal isolates, which produced distinct clearing zones around their colonies, were selected. Isolated fungi were identified using standard reference manuals [14] by wet mount preparation. Aspergillus niger isolates were preserved on PDA slants for further studies. The isolates were cultured on standard substrate like birch wood xylan. Protein content of the culture supernatant was assayed by Lowry’smethod using Bovine serum albumin as standard [16]. Further, the enzyme was partially purified by ammonium sulphate precipitation and dialysis. Partially purified Xylanase activity was assayed by DNS method using birch wood xylan (Fluka) as enzyme substrate. |
Xylanase assay |
| Xylanase activity was assayed by 3, 5-dinitrosalicylic acid method using birch wood xylan (Fluka) as enzyme substrate and xylose as standard. One unit of enzyme was defined as the quantity of enzyme that released 1 μmol of reducing sugar per minute [3].The amount of xylanase produced was quantified under submerged and solid state fermentation conditions using natural substrates like corn cob, rice husk and saw dust. |
Submerged fermentation (SmF) |
| The constituents of the media are dissolved in distilled water at 60ºC for 1hr, distributed to different conical flasks, sterilized by using autoclave and then inoculated with 10 discs (inoculum size 75 x 106spores. ml-1) of each isolate [12]. Above conical flasks are incubated for seven days on rotary shaker. The culture broth was filtered using whatmann filter paper and centrifuged at 6000 rpm for 15 minutes. The supernatant was collected for enzyme assay. The ability of isolates to produce xylanase enzyme was checked. Protein content of the culture supernatant was assayed by the method of Lowry using Bovine serum albumin as standard [16] and xylanase activity was determined by DNS method. |
Solid-state fermentation (SSF) |
| The isolates showing highest xylanase activity from SmF were selected for SSF method of xylanase production. Cultivation of fungus was performed in Erlenmeyer flask containing 10 g of solid substrate with the addition of 15 ml of Mandel’s medium [9]. The medium and the trace elements were autoclaved separately. The flask was cooled down at room temperature and a known amount of sterilized trace elements was added. The flasks were then inoculated and incubated for 5 or 7 days at the ambient temperature (28±3ºC).Seventy milliliters of cold distilled water (4ºC) was added to the SSF medium (10 g substrate) after cultivation. The mixture was vigorously homogenized for 30 minutes at 200 rpm. The solid biomass residues were separated from the suspension by filtration through Whatmann filter paper No.1. The cell free supernatant was used as the source of crude enzyme preparation. Protein content and Xylanase activity of the culture supernatant was assayed using natural substrates, as conducted in the submerged fermentation. |
Effectiveness of xylanase in Pulp treatment |
| The pulp from sugarcane after extraction of sugar is called sugarcane bagasse and is used alone or mixed with wood chips for making paper. The screened, unbleached pulp from sugarcane bagasse (10 % consistency) was incubated with crude culture filtrate at a ratio of 10g pulp to 1ml enzyme at 55°C for 1 h. After incubation, the pulp suspension was filtered through a Whatman No.1 filter paper and air-dried. The delignification was measured as change in kappa number which is indicative of the extent of delignification and bleachability of the pulp. The kappa number is the volume of 0.1N potassium permanganate solution consumed by 1g of moisture-free pulp under the conditions described in the standard procedure of TAPPI (Technical Association of Paper and Pulp Industries) Test method T236-cm-85 [1]. The kappa number x 0.15 gives the percentage of residual lignin [5]. |
IV. EXPERIMENTAL RESULTS |
Primary screening for isolation of Aspergillus niger |
| From the primary screening, five isolates were obtained from the soil samples of different places (Table 1). |
 |
| Identification of fungi was done based on colony characters and microscopic examination. After identification of Aspergillus niger, the spore count was done which is observed to be 75x105 for one disc of 5mm diameter from PDA plates, using haemocytometer. Xylanolytic activity is determined by using xylose calibration graph as standard. |
Secondary screening |
| From the primary screening, five isolates obtained were further confirmed for Xylanase production by secondary screening using congo red method. |
Determination of crudeenzyme activity |
| After inoculation and incubation of standard substrate with isolates, the supernatant obtained is the main source of xylanase. Protein content and xylanase activity of the supernatant were determined (Table 2). |
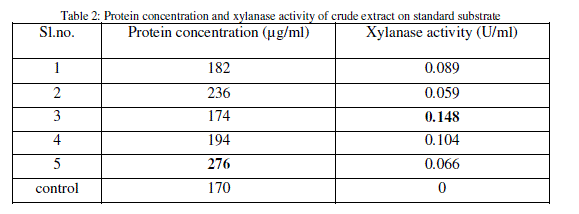 |
| The crude enzyme was further subjected to purification by ammonium sulphate precipitation and dialysis in order to obtain the partially purified enzyme solution. |
Determination of partially purified enzyme activity |
| The xylanase activity of the partially purified enzyme for all the substrates was determined by DNS method (Table 3). |
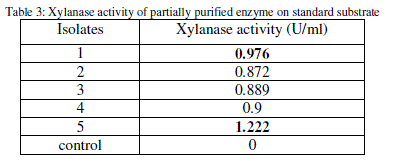 |
Submerged fermentation (SmF) |
| Several agricultural wastes like corn cob, rice husk and saw dusts were examined as substrates in comparison to birch wood xylan for the growth and xylanase production by the isolates through submerged fermentation, where corn cob showed highest xylanase activity (Table 4). |
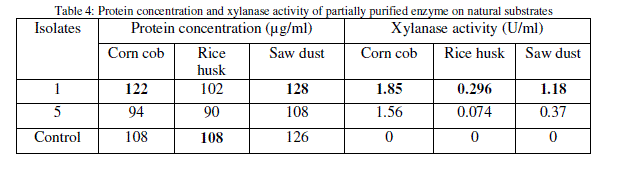 |
Solid state fermentation |
| Further, the study of solid state fermentation was carried out with the isolates 1 and 5, which have shown highest xylanase activity. |
| The protein content for each of the substrate was analyzed and it was found that the substrate corn cob is having much protein content than other two substrates. The xylanase activity was observed to be significant on all the substrates and it was found highest on corn cob than other substrates (Table 5). |
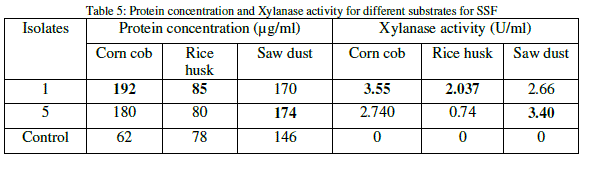 |
Comparision of xylanase activities of Submerged and Solid state fermentation. |
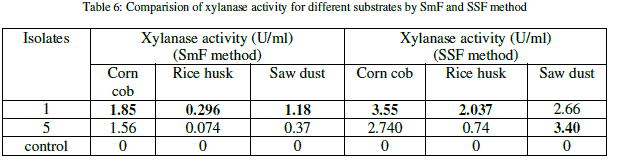
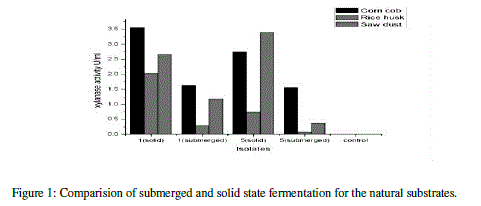
| The xylanase activity was observed to be highest in solid state fermentation compared to submerged fermentation. Among the natural substrates corn cob showed highest xylanase activity (Table 6) (Figure 1). |
 |
 |
Effectiveness of xylanase for Pulp treatment by determination of kappa number |
| The partially purified xylanase of the isolate 1 was tested for its biobleaching potential. The culture filtrate of this isolate containing 0.22 U.ml-1 of enzyme could bleach pulp by a 60 minutes treatment at 55°C, resulting in 20 point reduction in the kappa numbers. The unbleached pulp showed 73kappa number and the enzyme-treated pulp showed a kappa number of 52.57. The percentage lignin for untreated pulp is 10.95% and for enzyme treated was 7.8855%. The isolate 5 showed 13 point reduction in the kappa number. The enzyme treated pulp showed a kappa number of 60.78. The percentage lignin for enzyme treated pulp was 9.117%. |
V. DISCUSSION |
| Xylan is the major component of hemicellulose which is the second most abundant polysaccharide. Degradation of xylan into sugars is of much importance for many industries and so is the Xylanase enzyme required for its degradation. |
| Screening for isolating xylanase producing Aspergillus niger was carried out on the xylan medium. Among the twelve fungal colonies obtained, five were identified to be Aspergillus niger by the colony characteristics and microscopic examination. The congo red test was positive by forming reddish orange halo-zone of hydrolysis which confirmed that they were xylanase producing isolates. |
| Xylanase activity was determined from the supernatant obtained after submerged fermentation of the standard substrate birch wood xylan by the five isolates. |
| The use of purified xylan enhances the cost of enzyme production and is a major limitation to the economic feasibility of the enzyme. The use of agricultural residue is not only inexpensive, but also it is abundant and easily available, supplying the microorganism better nutrition [4]. By testing the xylanase production on various substrates, it was found that the enzyme was active on natural substrates like corn cob than the standard substrate like birch wood xylan. These results showed that this xylanase was specific for hydrolyzing natural xylan, which is a desirable property for industrial applications like bleaching of pulps, where it results in better porosity, swelling up and separation of pulp microfibrils and pulp fibers [2]. |
| To select the best fermentation condition for xylanase production by A.niger, SmF and SSF was carried out. The higher xylanase production was achieved in SSF than in SmF. Although a number of xylanase productions were performed using submerged systems, solid state fermentation was found to be more economical mainly due to the cheap and abundant availability of agricultural wastes which can be used as substrates. The choice of the substrate was mainly on the basis of percentage of cellulose and hemicelluloses content. Besides that SSF offers distinct advantages over submerged fermentation including economy of space needed for fermentation; simplicity of the fermentation media; no requirement for complex machinery, equipment and control systems; greater compactness of the fermentation vessel owing to a lower water volume; greater product yield; reduced energy demand; lower capital and low recurring expenditures in industrial operation; easier scale up processes; lesser volume of solvent needed for product recovery; superior yields; absence of foam build up; and easier control of contaminants due to the low moisture level in the system [13]. |
| Effectiveness of the partially purified enzyme was determined by pulp treatment which is one of the industrial applications of xylanase. The drop in kappa number after biobleaching indicates reduction in chlorine consumption during further bleaching of the pulp. The kappa number is a measure of delignification that the raw material has experienced. High values of kappa number imply that the pulp may not be well suited for carrying out bleaching of the pulps because of high cost [7]. The kappa number obtained for enzyme treated pulp showed twenty point reduction than untreated pulp. The percentage lignin content was also less (two point reduction) for enzyme than untreated pulp. Hence, this enzyme (from isolate 1) can be used in the treatment of pulp industrially. Hence, the isolates obtained can be used for production of xylanases on natural substrates and they can be ecofriendly when used in industrial applications. |
VI. CONCLUSION |
| This study highlights the use of natural substrates for the production of xylanase by submerged and solid state fermentation, which is cost effective and economically benefitted, when used in industrial processes. |
References |
|