E- ISSN: 2320 - 3528
P- ISSN: 2347 - 2286
E- ISSN: 2320 - 3528
P- ISSN: 2347 - 2286
1Drug Delivery System Excellence Center, Faculty of Pharmaceutical Sciences, Prince of Songkla University, Hat Yai, Songkhla, 90112, Thailand
2Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Prince of Songkla University, Hat Yai, Songkhla, 90112, Thailand
Received date: 05/03/2019; Accepted date: 14/03/2019; Published date: 22/03/2019
Visit for more related articles at Research & Reviews: Journal of Microbiology and Biotechnology
Microencapsulation is widely used to stabilize probiotics to allow their full functioning. The aim of the study was to determine the optimal combination of Bambara groundnut protein isolate (BGPI)/alginate and inulin as the encapsulating agents for the probiotic; L a c t o b a c ill u s r h a m n o s u s GG was established using response surface methodology (RSM). The probiotic cells were encapsulated in various ratios of BGPI/alginate-inulin wall according to RSM with complex coacervation technique. It was found that the optimal concentration level of 1:1 weight ratio BGPI/alginate solution was 2.14% w/v with 3.23% w/v inulin. The formulation yielded 96.64% encapsulation efficiency, having survival rate as high as 95.76% and 94.48% after freeze drying process and after exposure to acid condition respectively. Not only was the validity of the in-use predicted model confirmed but capsules also improved the survival rate of the cells in simulated gastric fluid (SGF) by ∼ 4.88 log CFU/ml after 3 h compared with free cells and demonstrated the cell release of 8.53 log CFU/ml within 4 h. The survival rate of entrapped cells stored at 4°C and 30°C for 6 months (7.82 log × FU/ml and 8 log CFU/ml, respectively) were superior to those of free cells in the identical conditions, (4.10 log CFU/ml and <1 log CFU/ml respectively). Encapsulation of probiotics in BGPI/alginate-inulin capsules offers opportunities for improving the viability of cells during freeze drying process, exposure to the acidic medium of the stomach and storage.
Lactobacillus rhamnosus GG, Complex coacervation, Bambara groundnut protein isolate, Gastrointestinal tract, Freeze drying
Probiotics play a major role in food and pharmaceutical industry as they aim to increase their nutritional and therapeutic values [1]. The advantages for human health associated with probiotics intake include control of intestinal infection, reduction of serum cholesterol levels, suppression of cancer, improved digestion, reduction of the symptoms of lactose malabsorption and stimulation of gastrointestinal immunity [2-4]. The positive health effects of probiotic have, in turn, stimulated demand for functional probiotics in food products in recent years. It is recommended that food containing probiotic bacteria should contain at least 106 live microorganisms per gram or milliliter at the time of consumption [5,6]. However, available probiotic products often provide the low cell populations because the viable cells encountered the harsh conditions during manufacturing process, storage and passage through the human gastrointestinal tract [7]. The maintenance of viability of these bacteria at sufficiently high levels to confer beneficial effects has provided a major challenge to formulation scientists in the pharmaceuticals and foods industries [8]. In response, probiotic cells have frequently been shielded against environmental conditions by microencapsulation in a variety of polymers using a number of techniques including extrusion, emulsification and spray drying [9,10]. Complex coacervation, in particular, has attracted widespread interest since the technique is easily implemented and is relatively low cost [11]. Complex coacervation involves the precipitation of two or more biopolymers from solution, for example a protein and ionic polysaccharide of opposite charge, leading to phase separation. Subsequent deposition of the formed coacervate occurs around incorporated solid particles or liquid droplets, forming a surface layer or coating [12]. For example, whey protein isolate and ҡ-carrageenan, an anionic polysaccharide, were successfully used for encapsulation of Lactobacillus plantarum and the obtained microcapsules were amenable to drying using a variety of techniques [13].
Varieties of natural polymers such as alginate, pectin, carrageenan, gum arabic and gelatin have been used as encapsulating materials [14,15]. Sodium alginate is widely-used in the food industry as a carrier for probiotics and prebiotics because of the polymer’s biocompatibility, controllable biodegradability, range of physicochemical properties and low cost [16-18]. More recently, proteins including whey protein, pea protein, gelatin and casein have received increasing attention as a potential alternative encapsulating material for probiotic bacteria [16,19,20]. The interest in legume proteins for encapsulating various entities in the food industry stems from their food functionality and low cost [21]. Legume proteins derived from peas, chickpeas and soy have previously been combined with polysaccharides to encapsulate and protect probiotic cells from harmful environments encountered in manufacture and following oral administration [22,23].
Bambara groundnut legume or Vigna subterrnanea (Leguminosae) is found in the southern part of Thailand, the African continent, Brazil, as well as Western Java [24,25]. Recently, Bambara groundnut milk and protein isolated from this legume can also be used with lactic acid bacteria to make an advantage for probiotic product that not only increase the economic value of the nutritious legume but also improve the viability of probiotic from harmful environments [26,27]. Prebiotics are non-digestible food ingredients that stimulate the growth or activity of bacteria in the gastrointestinal tract. Certain prebiotics, such as inulin, fructo-oligosaccharides and resistant starch have also been used as a co-encapsulating material to increase the viability of bacteria which are exposed to acidic conditions during storage or following ingestion [28,29].
Only a few studies have focused on the advantages of legume protein isolates and prebiotics for encapsulation of probiotics. Thus, the combination of legume proteins and prebiotics is of interest for simultaneously improving protection of probiotics and stimulating the activity of both delivered and resident bacteria in the gastrointestinal tract. Optimisation of the composition of encapsulating materials and the process is necessary to maximize encapsulation efficiency and the viability of cells during freeze-drying and exposure to the acidic conditions of the gastrointestinal tract. The classical experimental approach varies one-parameter at a time, which is time-consuming and does not include interaction among the variables or depicts the complete effects of the parameters on the process. Over the last two decades, Response Surface Methodology (RSM) has gained increasing acceptance as an efficient statistical technique for investigating the effect of various factors on a process. Several formulation parameters are varied simultaneously in order to predict the optimum process conditions based on a minimum number of experiments [30].
The objective of the present study was to determine the optimum combination of BGPI/alginate and inulin for encapsulation of Lactobacillus rhamnosus GG using the complex coacervation technique. The optimised formulation was expected to maximize the encapsulation efficiency, the survival rate of encapsulated cells after freeze drying and storage and during exposure to simulated gastric fluid and in simulated intestinal fluid.
Bacterial Strain
A commercial probiotic strain of Lactobacillus rhamnosus GG (ATCC 53013) was purchased from the American Type Culture Collection. The stock cultures were preserved in De Man, Rogosa, and Sharpe (MRS) broth (Difco, Sparks, MD, USA) with 25% w/v glycerol at -80°C. Prior to use, frozen cultures were activated by culturing in MRS broth on two successive incubations in anaerobic conditions maintained in an anaerobic jar containing a gas-pak microbiology Anaerocult® A (Merck, Darmstadt, Germany) at 37°C for 48 h.
Preparation of L. rhamnosus GG-loaded Microcapsules by Complex Coacervation and Preparation of Bambara Groundnut Protein Isolate
Mature Bambara groundnut seeds were obtained from a local market, HatYai, Songkhla, Thailand. The sample (1 kg) was dehulled and ground using a grinding machine (Health Herb Products Co., Ltd., Thailand) to obtain a fine powder. Bambara groundnut protein isolate (BGPI) was prepared according to the methods of Pastor-Cavada et al. [31] with a slight modification as follows. Twenty grams of groundnut powder was suspended in 100 ml of 2 g/l NaOH solution (pH 12). The mixture was stirred continuously for 2 h at room temperature (30°C) followed by centrifugation at 6000 × g for 30 min at 25°C using a Beckman Model Avanti J-E centrifuge (Beckman Coulter, Inc., Fullerton, CA, USA). The supernatant was collected and adjusted to pH 4.5 using 6 M HCl where upon a precipitate was formed and subsequently isolated by centrifugation at 6000 × g for 30 min at 25°C. The resulting pellet was washed with 10 volumes of distilled water (pH 4.5) followed by centrifugation at 8000 × g for 30 min and using a freeze dryer (model FD-300 Airvac Engineering Pty Ltd., Dandenong, Australia) at a condenser temperature of -40°C for 20 h. The dried powder was referred to as ‘Bambara groundnut protein isolate (BGPI)’. BGPI was placed in sealed polyethylene bag and stored at 4°C until use.
Encapsulation Procedures
The probiotic cells were encapsulated by BGPI/alginate (1:1 weight mixing ratio) and inulin using the complex coacervation technique. The BGPI/alginate and inulin solution were prepared in various concentrations as generated by the experimental design described below. The encapsulation process was performed by adding the cell dispersion in 2 ml inulin solution (cell density 109-1010 CFU/ml) into 8 ml BGPI/alginate solution and the mixture was stirred gently for 20 min. The pH of the mixture was subsequently adjusted to pH 4.5 by slowly adding a 10% (v/v) lactic acid solution to form coacervates and left to stand at room temperature (30°C) for at least 30 min to allow complete phase separation. Subsequently, the complex coacervation phase in the form of microcapsules was obtained by decantation of the clear supernatant. The microcapsules were washed three times with deionized water (pH 4.5) and then freeze-dried with the conditions as described previously. The number of viable encapsulated cells before and after the freeze-drying process was enumerated to calculate a survival rate.
Experimental Design
The desired results of responses, i.e., entrapment efficiency (EE, %), viability of probiotic cell in acid solution pH 2.0 (%) and viability of the cells after freeze drying (%) were required. Two main factors includes BGPI/alginate aqueous solution of 1-3% w/v prepared with biopolymer mixing ratio of 1:1 and inulin solution of 1-5% w/v. As shown in Table 1, thirteen different combinations were prepared for the optimization procedure based on a central composite design (CCD) to investigate the effect of two factors on the responses listed above.
| Trial no. | Factor (%) | Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| Encapsulation efficiency (%) | Survival rate (%) in acid condition | Survival rate (%) after Frreze-drying | ||||||
| BGPI/alginate | Inulin | |||||||
| Experimental | Predicted | Experimental | Predicted | Experimental | Predicted | |||
| 1 | -1 (1) | -1 (1) | 85.18 | 86.01 | 82.45 | 82.79 | 83.22 | 83.9 |
| 2 | 1 (3) | -1 (1) | 89.5 | 89.75 | 87.76 | 87.77 | 88.74 | 88.96 |
| 3 | -1 (1) | 1 (5) | 89.12 | 89.41 | 87.35 | 87.06 | 89.35 | 88.67 |
| 4 | 1 (3) | 1 (5) | 89.81 | 89.53 | 88.98 | 88.36 | 89.59 | 88.46 |
| 5 | 1.4142 (0.58) | 0 (3) | 87.43 | 86.68 | 84.27 | 84.09 | 85.49 | 85.32 |
| 6 | 1.4142 (3.41) | 0 (3) | 89.35 | 89.53 | 88.24 | 88.65 | 88.28 | 88.87 |
| 7 | 0 (2) | 1.4142 (0.17) | 88.75 | 88.09 | 85.18 | 84.87 | 87.11 | 86.37 |
| 8 | 0 (2) | 1.4142 (5.38) | 90.25 | 92.32 | 87.75 | 90.29 | 88.2 | 91.41 |
| 9 | 0 (2) | 0 (3) | 95.93 | 96.56 | 93.27 | 94.26 | 94.23 | 95.62 |
| 10 | 0 (2) | 0 (3) | 96.9 | 96.56 | 94.14 | 94.26 | 95.98 | 95.62 |
| 11 | 0 (2) | 0 (3) | 95.9 | 96.56 | 93.89 | 94.26 | 94.34 | 95.62 |
| 12 | 0 (2) | 0 (3) | 97.2 | 96.56 | 95.24 | 94.26 | 96.39 | 95.62 |
| 13 | 0 (2) | 0 (3) | 96.85 | 96.56 | 94.76 | 94.26 | 96.17 | 95.62 |
Table 1: Treatment combinations of encapsulation agents bambara groundnut protein isolate (BGPI)/alginate and inulin according to the central composite design with experimental and predicted values on encapsulation efficiency, survival rate after exposure to acid conditions and after freeze drying process of L. rhamnosus GG.
The statistical significance of the model was determined by F-test, and the analysis of variance (ANOVA) for the fitted quadratic polynomial model was performed. The design of experiments as well as the analysis of data and optimization procedure was performed using Design Expert software (Trial version 9.0.3.1; Stat-Ease, Minneapolis, USA; www.statease.com).
Regarding to the accuracy of the predicted model, the adequacy of the regression equation of the experimental data and the predicted values were determined using the R2 and absolute average deviation (AAD) which was calculated as:
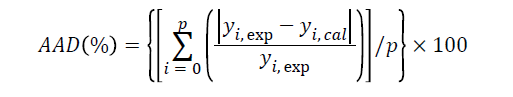
where yi,exp and yi,cal are the experimental and calculated responses, respectively and p is the number of experimental runs.
Enumeration of Free Cells and Encapsulated Cells
The encapsulated microcapsules were added into a 0.1 M phosphate buffer solution (pH 7.4), the mixture was vigorously stirred for 30 min at room temperature so as to break the polymer wall and to completely release the cells into the buffer. Certain sequential dilutions were made to achieve countable cells numbers. The bacterial cells were plated on MRS agar at 37°C under anaerobic conditions for 48 h, while free cells were also enumerated similarly.
Determination of Encapsulation Efficiency
The encapsulation efficiency (%) was calculated as follows:
Encapsulation efficiency (%)=[log N/log N0] × 100
where N is the number of cells released (for enumeration) from the total coacervated mass and N0 is the initial number of cells added to the mixed polymeric solution used for microencapsulation.
Effect of Encapsulation Matrix on Cell Viability in Acid Solution pH 2.0
The protective effect of encapsulation matrix on cell viability in acid solution was determined by the method suggested by Lee and Heo [32] as follows. Free cells and encapsulated cells were added into the acid solution containing 0.2% w/v NaCl at pH 2 (adjusted by 0.1 N HCl) at 37°C for 2 h.After incubation, the acid solution was immediately decanted and the pellets were then washed twice with sterilized normal saline solution to relieve acid stress.The free cells and released bacterial cells from microcapsules were enumerated by the method described above.
Light and Scanning Electron Microscopy of Encapsulated Cells
The fresh samples of microcapsules were transferred on glass slides and then examined under light microscope (Olympus, Tokyo, Japan). Further morphological information was obtained using Scanning Electron Microscopy (JSM-5800LV, JEOL, Japan). Freeze-dried samples were mounted on aluminium stubs using double-sided tape and sputter coated with gold, prior to observation at 15 kV.
Survival of Free Cells and Encapsulated Cells in Simulated Gastric Fluid
Simulated gastric fluid (SGF) was prepared according to the method described by Gbassi et al. [33] with modifications. Normal saline solution at the concentration of 9 g/l was prepared, and the pH was adjusted to 2.0 with 1 M HCl. The solution was then sterilized by autoclaving at 121°C for 15 min. Pepsin (Sigma-Aldrich, USA) was added to the concentration of 3.0 g/l and the solution was filtered through 0.22 μm sterile membrane. Free cells or encapsulated cells were transferred into SGF and incubated at 37°C under orbital shaking at 160 rpm for 3 h. After the incubation, samples were removed at 0, 30, 60, 120 and 180 min and viable bacteria were enumerated according to the method described above. The percent survival of free cells and encapsulated cells was compared among sampling intervals.
Release Studies within Simulated Intestinal Fluid
Release profile of encapsulated L. rhamnosus GG in BGPI/alginate-inulin microcapsules was studied in simulated intestinal fluid (SIF). The SIF was composed of 1 g/l pancreatin (Sigma-Aldrich, USA) and 3 g/l bile salts (Sigma-Aldrich, USA) in 12.5 g l-1 of NaHCO3 and its pH was adjusted to 7.0 with 1 M NaOH [34]. The solution was filtered through 0.22 μm sterile membrane. BGPI/alginate-inulin microcapsules containing L. rhamnosus GG were added to conical plastic tubes containing pre-warmed SIF and incubated at 37°C with shaking at 160 rpm. Aliquots (100 μl) of release medium were collected at time intervals 0, 30, 60, 120, 240 min and replaced with an equal volume of fresh medium. The withdrawn samples were immediately assayed for the number of viable L. rhamnosus GG bacteria released according to procedures described above and the cumulative release was plotted against time.
Survival of Encapsulated Cells during Storage
The free and encapsulated probiotic cells (400 mg) in freeze-dried form were manually packed into gelatin capsules No.1. The capsules were placed in an air-tight amber glass bottle containing silica gel desiccant packed down tightly with a wad of cotton wool. The storage stability of free and encapsulated L. rhamnosus GG was tested at 4°C and 30°C at one month intervals for 6 months by assay of the viability of free and encapsulated cells according to the method described above.
Developing and Checking the Fitted Model
Response surface methodology was applied to study the influences of wall concentration (BGPI/alginate) and the amount of inulin on encapsulation efficiency of probiotic cells, and survival rate in the harsh environments including in acid condition and after freeze drying. The single factor experiment with less than 1% w/v wall concentration showed that there were not only low microcapsule yields but also low encapsulation efficiency as well as low in-acid and after-freezedrying survival rates. On the other hand, the higher BGPI/alginate concentration (>3% w/v) resulted in irregular microcapsule morphology, large particle size, and agglomeration of microcapsules that is difficult for further process. Then, it was not recommended for preparation and further application of these probiotic-loaded microcapsules. The concentration of inulin was chosen from the results of other studies [35,36]. Thus, the wall concentration (BGPI/alginate) and inulin should be optimized within the ranges of 1-3% w/v and 1-5% w/v respectively.
To optimize the probiotic-loaded coacervate microcapsules, thirteen experiments were designed by CCD in RSM. The experimental and predicted responses for encapsulation efficiency of probiotic cells, survival rate in acid condition and after freeze drying are presented in Table 1. Relationships between the two independent variables and three responses were given by the following regression equations:
Encapsulation efficiency
Y1=65.5982+19.1932A+6.8048B–0.4533AB–4.2170A2–0.9165B2
Survival rate of probiotic in acid condition
Y2=62.1788+18.6950A+7.2726B–0.4595AB–3.9366A2–0.9574B2
Survival rate of probiotic after freeze-drying
Y3=61.9174+20.2120A+7.6579B–0.6597AB–4.2550A2–0.9676B2
Where Y1, Y2 and Y3 (%) are encapsulation efficiency, the cell survival rate of L. rhamnosus GG in acid condition and after freeze-drying process, respectively and A and B are the concentration level variables of BGPI/alginate and inulin, respectively.
Significance test of regression model, individual model coefficients and lack of fit were carried out with ANOVA. The results of ANOVA and lack of fit tests with correlation coefficients are showed in Table 2. Systematically, the fitness of the model has been governed by the significance of the model (p<0.05) and the insignificance of lack of fit (p>0.05). And, it is indicated by the coefficient of determination, R2, which measures the percentage of response variables that can be accounted for or explained by independent variables, as outlined by the previous studies [32,37]. For a good fit of a model, Joglekar & May [38] suggested that R2 values should be at least 0.80.
| Reponses | Source | Sum of square | Degree of freedom | Mean square | F value | p value |
|---|---|---|---|---|---|---|
| Encapsulation | Regression | 208.26 | 5 | 41.65 | 89.21 | 0.0001 |
| efficiency (%) | Residual | 3.27 | 7 | 0.47 | - | - |
| Lack of fit | 1.82 | 3 | 0.61 | 1.67 | 0.309 | |
| Pure error | 1.45 | 4 | 0.36 | - | - | |
| Total | 211.53 | 12 | - | - | - | |
| Survival rate (%) in acid condition | Regression | 220.59 | 5 | 44.12 | 88.51 | 0.0001 |
| Residual | 3.49 | 7 | 0.5 | - | - | |
| Lack of fit | 1.14 | 3 | 0.38 | 0.65 | 0.6234 | |
| Pure error | 2.35 | 4 | 0.59 | - | - | |
| Total | 224.08 | 12 | - | - | - | |
| Survival rate (%) after freeze-drying | Regression | 231.55 | 5 | 46.31 | 43 | 0.0001 |
| Residual | 7.54 | 7 | 1.08 | - | - | |
| Lack of fit | 4.51 | 3 | 1.5 | 1.98 | 0.2588 | |
| Pure error | 3.03 | 4 | 0.76 | - | - | |
| Total | 239.09 | 12 | - | - | - |
Table 2: Analysis of variance (ANOVA) for evaluation of the second-order response surface model.
Effect of Formulation Variable on Probiotic Cell Encapsulation Efficiency
Response surface plot relating entrapment efficiency is presented in Figure 1A. The 2 (0.9845) was high indicating the adequate fitting of the quadratic model. The magnitude of coefficient as well as the sign it carries, i.e., positive or negative in polynomial regression made one to draw the significance of the variables. The viability of the encapsulated L. rhamnosus GG was mostly affected by the BGPI/alginate (A), while inulin (B) concentration had the lowest influence. In the interaction case, the encapsulation efficiency increased with increases in concentration of BGPI/alginate and inulin until a maximum level was attained. Further increase in BGPI/alginate beyond 2.14% w/v only had a subtle effect on encapsulation efficiency. Similar to the effect of BGPI/alginate concentration, increases in inulin concentration increased the encapsulation efficiency, but the rate of increase was rather subtle when the concentration of inulin higher than 3.23% w/v.
Effect of Formulation Variable on the Viability of L. rhamnosus GG in Acid Condition
The three-dimensional response surface plot relating viability in acid condition is presented in Figure 1B with good coefficient of determination (R2 value of 0.9837). Analysis of these parameters indicates that BGPI/alginate was the most effective factor on the viability of L. rhamnosus GG in acid condition followed by inulin. The figure shows the effect of different concentrations of BGPI/alginate and inulin on the mean values of the viability of probiotic in acid conditions. The highest cell viability was obtained when the mean values of BGPI/alginate and inulin were used.
Effect of Formulation Variable on the Viability of L. rhamnosus GG after Freeze-drying
The effect of concentrations of BGPI/alginate and inulin on the mean values of viability of L. rhamnosus GG after freeze drying is presented in Figure 1C. It was evident that the cell viability of freeze dried L. rhamnosus GG steadily increased with increasing the concentration of BGPI/alginate and inulin up to their mean value, but decreased beyond theses concentrations. The coefficient of determination (R2) of 0.9685 was obtained.
Verification of Predictive Models
To determine the validity of the statistical model, treatments of protective material in the optimal concentration of variables including BGPI/alginate 2.14% (w/v) and inulin 3.23% (w/v) was applied. Under the optimized conditions the predicted survival rate of L. rhamnosus GG for encapsulation efficiency, survival rate in acid condition and that after the freeze-drying process was 96.64%, 94.48% and 95.76% respectively. The observed experimental values of the responses under optimum condition of process parameters were 95.92% encapsulation efficiency, 93.69% survival rate after acid condition and 96.21% survival rate after freeze-drying process. These results confirmed the validity of the model and showed that the experimental values were in a good agreement with the predicted values. Deviation from the mean were calculated as 0.75%, 0.84% and 0.46% for encapsulation efficiency, survival rate in acid condition and survival rate after the freeze-drying respectively. The experimental values, mean of three trials, were found to be in close agreement with the predicted values and were within the acceptable limits which showed the adequacy of selected models.
Morphology Analysis
As seen in Figure 2A, complex coacervation resulted from the interaction of BGPI and alginate with opposing charges, giving rise to the formation of macromolecular networks which entrapped inulin and L. rhamnosus GG cells. Optical microscopy revealed the fairly uniform spherical shape of L. rhamnosus GG containing microcapsules prepared by complex coacervation, which ranged from 124 to 352 μm. The morphology of freeze dried microcapsules by scanning electron microscope is presented in Figure 2B indicated that high agglomeration, leading to a loss of spherical shape and producing a flake like structure with a variety of sizes. In the higher magnification (5000x), Figure 2C revealed that there were bacterial cells embedded on the surface and inside of microcapsules.
Survival of Microencapsulated L. rhamnosus GG Cells in Simulated Gastric Fluid
Certain bacteria operate a defense mechanism in acid environments whereby a proton pump or proton/cation exchange system in the plasma membrane compensates for the influx of protons to maintain the cytoplasm near neutral pH. However, in highly acidic conditions the cell’s pH regulatory mechanism is unable to function sufficiently and the resulting intracellular acidification leading to loss of viability. When free L. rhamnosus GG cells and cells encapsulated in BGPI/alginate and inulin by complex coacervation under optimized formulation conditions were exposed to simulated gastric fluid (SGF, pH 2.0) at 37°C, a major reduction in the number of non-encapsulated cells was measured following 3 h treatment (log reduction of 5.98 log CFU/ml). In contrast, a fairly minor loss of viability resulted (1.05 log CFU/ml) for encapsulated cells as shown in Figure 3, in line with the work of Sheu and Marshall [39], Lee and Heo [32] and Chandramouli et al. [40]. The present findings highlight the marked deleterious effect of the gastric environment on unprotected probiotic cells and the efficiency of the developed complex coacervation-based microencapsulation technique in shielding cells from hostile, external environments. L. rhamnosus GG appears to be efficiently enclosed by the coacervates produced by interaction between BGPI/alginate and inulin. In the acid environment of SGF the ionizable groups of the proteins (BGPI) are protonated and the dissociated carboxyl groups of the polysaccharide (alginate) are diminished, thus the cells are likely to be located in a compact polymeric matrix which provides a highly effective shield against the extreme pH of SGF. In addition, the presence of inulin as a prebiotic in the microcapsule matrix may improve protection of probiotic cells by physically blocking the pores of the matrix, thus impeding diffusion of SGF into the capsule. The protective effect of inulin towards alginate-encapsulated probiotic bacteria exposed to gastric conditions was reported previously [41].
Release Characteristic of the Encapsulated L. rhamnosus GG in Simulated Intestinal Fluid
Efficient delivery and release of encapsulated probiotic cells in the small intestine and colon is essential for growth and colonization confer healthy function. The release profile of encapsulated L. rhamnosus GG in simulated intestinal fluid (SIF, pH 6.8, 37°C) containing pancreatin, over a 4 h time period is showed in Figure 4.
The number of viable cells measured at 30 min totaled around 6.68 log CFU/ml and the number gradually increased over time, reaching approximately 8.53 log CFU/ml by 4 h. The cell release mechanism may be explained by swellingerosion of the BGPI-alginate network in SIF, accelerated by the sodium ions (Na+) in the release medium, which may act to destabilize the protein-alginate network. Smidsrod and Skjakbraek [42] demonstrated that the presence of sodium ions results in rapid breakdown of alginate hydrogels, crosslinked by Ca2+, by a process of ion exchange. In addition, the pancreatin present in SIF may digest the BGPI and accelerate breakdown of the microcapsules. Free L. rhamnosus GG cells displayed a reduction in viability from 9.49 log CFU/ml to 7.34 log CFU/ml after 4 h, probably due to the loss of cell wall integrity as a result of exposure to bile salt in the SIF release medium. Klemmer et al. [23] previously reported that encapsulated probiotic cells showed higher survival rates than free cells in SIF containing bile salt.
Storage Stability of Free and Encapsulated L. rhamnosus GG
The survival rates of free and encapsulated L. rhamnosus GG cells in BGPI/alginate-inulin microcapsules during 6 months storage at 4°C and 30°C are indicated in Figures 5A and 5B respectively. At 4°C storage temperature (Figure 5A), the survival numbers of free cells decreased from 9.10 to 4.10 log CFU/ml after 6 months storage. On the other hand, there was slight decrease in encapsulated cells from 9.55 to 7.82 log CFU/ml under the same conditions. According to the results shown in Figure 5B, in control group containing free bacteria, the cell counts decreased during storage, with a reduction of 4.26 log CFU/ml at 30°C after 4 months of storage, and none was counted after 6 months. In the case of BGPI/alginate-inulin, encapsulated bacteria showed only a ~ 3 log decrease in cell numbers after 6 months. High reduction of free cells during storage may be due to the effect of ice crystals formation during freeze drying process on structural damage with ruptures of the membrane. Changing physiological state of the cells leads to increase the rate of fatty acid oxidation during storage. In the case of encapsulated cells, the encapsulated materials i.e., BGPI, alginate and inulin may protect the cell from the harmful effect of ice crystals during freezing. Furthermore, encapsulated wall forms a barrier against oxygen whereas sulfur-containing amino acids present in BGPI could contribute to the maintenance of low redox potentials and act as oxygen scavengers leading to the improved cell survival during storage. Hugo et al. [43] were also reported that the survival rate of probiotic cells encapsulated in a soy protein isolate with a calcium chloride matrix was higher than free cells during storage. These results, therefore, suggest that BGPI/alginate-inulin microcapsules are promising system to improve viability of probiotic cells during storage.
The probiotic L. rhamnosus GG was successfully encapsulated in BGPI/alginate-inulin using complex coacervation technique. The validation experiment demonstrated that RSM was reliable in developing a model, optimization of factors, and analysis of interaction effects. The optimum comprised 2.14% (w/v) BGPI/alginate co-solution (1:1 w/w) and 3.23% (w/v) inulin solution. The microcapsules prepared under the optimal conditions yielded the capsules with greater cellencapsulation efficiency, excellent protection of the encapsulated cells from the harmful effect of freeze drying process, gastric conditions and storage conditions compared with unprotected free cells. The microcapsules also efficiently released the probiotic cells in simulated intestinal fluid. Therefore, production of microcapsule of probiotic with the combination of BGPI/alginate and inulin wall matrix by complex coacervation technique has potential applications in pharmaceutical and functional food industry.
This research was supported in part by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission and Faculty of Pharmaceutical Sciences, Prince of Songkla University, Hat Yai, Songkhla, Thailand. We express our thanks Dr. Allan Coombes for his suggestions and corrections to the English.
All authors have no conflict of interest to declare.