E- ISSN: 2320 - 3528
P- ISSN: 2347 - 2286
E- ISSN: 2320 - 3528
P- ISSN: 2347 - 2286
Maryam Abbasi Gaznagh1, Asghar Khosrowshahi Asl2, Mahmoud Bahmani3, and Fariborz Kianpour4*
1Food and Hygiene Control Laboratory, Deputy for Food and Drug, Urmia University of Medical Sciences, Urmia, Iran
2Department of Food Science and Technology, Urmia University, Urmia, Iran
3Lorestan University of Medical Sciences, Khorramabad, Iran
4Department of Clinical Microbiology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
Received date: 16/06/2014; Accepted date: 19/07/2014
Visit for more related articles at Research & Reviews: Journal of Microbiology and Biotechnology
Jug (pot) cheese is a kind of delicious, semi-hard, and salty cheese in Azerbaijan region, Iran, which is traditionally produced from raw sheep's or cow's milk in rural households and then spends its ripening period under soil, which varies from 3 to 6 months. Given that this type of cheese is traditional and originally from Azerbaijan region, in this study for the first time, nitrogen fractions such as total nitrogen, non-casein nitrogen, and non-protein nitrogen were measured during the ripening of jug cheese. In this work, first, the cheese was ripened in two ways: being storing for 45 days in brine at 10°C and being storing for 90 days without brine in the 7±1°C fridge. Results showed that all nitrogen fractions increased during the cheese ripening. Moreover, concentration of tyrosine amino acid as a ripening indicator was determined by preparing of tyrosine standard curve and measuring of its absorption at the wavelength of 650 nm by a spectrophotometer device during the ripening period. It was observed that concentrations of this amino acid gradually increased during ripening, which represented the progress of proteolysis during this period. Also, effect of milk pasteurization on nitrogen fractions was examined and it was concluded that milk pasteurization had a small effect on these factors.
Jug cheese, Nitrogen fractions, Azerbaijan, Total Nitrogen, Non-casein nitrogen, Non-protein nitrogen.
Cheese is one of the most widely consumed dairy products and, depending on its type, has special flavors and fragrances, contains different amounts of milk ingredients including protein, fat, water, minerals, and vitamins, and is used daily, especially at breakfast [1,21]. Jug cheese (pot cheese) is a kind of tasty cheese made in Azerbaijan region. For its preparation, cheese is rammed in a special type of jar in the summer and, after tightening the lid, it is buried in the soil until the winter time. This cheese is semi-hard and salty which is traditionally prepared from raw sheep's or cow's milk in rural households in West Azerbaijan Province, Iran, and then spends its ripening period from 3 to 6 months under the soil. In fact, before transferring the cheese to the soil, it is left in 8% brine for 45 days in order to destroy brucellosis agent. Depending on the preferences and tastes of consumers, various additives such as cumin, pepper, thyme, oregano, acanthus, and different types of aromatic wild vegetables are added to it. It is also called cow or sheep jug cheese, Boukan cheese, Mahabad cheese, Maku cheese, acanthus cheese, mud cheese, cumin cheese, celery cheese, Sirak cheese, etc. Many studies have been done in Iran on cheese (milk and its products; 1.); but, there has been no works on jug cheese, as the traditional cheese of West Azerbaijan region from the point of its ripening. Objective of this study was first to assess the nitrogen fractions produced during traditional cheese ripening and second to investigate impact of milk pasteurization on the mentioned factors along with flavor and taste of the cheese.
Preparing of milk samples
Whole cow's milk (3.7% fat) was obtained from the animal husbandry of Urmia University. Fungal rennin with brand of Mito was provided from the market. Feta cheese mesophile starter which is a mixture of Lactobacillus delbrueckii subsp. Bulgaricus and Lactococcus lactis was obtained from Nooshinshahr cheese-making factory, Urmia, Iran. Mineral salt was used for brining the cheese and automatic Micro-Kjeldahl and spectrophotometer devices were used for doing the experiments. In this study, two methods were used for producing jug cheese. In the first method which was traditional, milk temperature was increased to 35°C after filtering and then rennet was added. After milk coagulation which took 45 min, curd cutting operation was done and, afterwards, the curds were left for 45 min so that their walls would be formed. The sample was stirred for 10 min and then whey was extracted from the curd. Cruds were placed in the mold and, after 3 h of pressure operation, the cheese was cut and put inside 25% saturated brine. After 24 h, it was taken out of the brine and was maintained outside at 10°C for 24 h. Then, the sample was transferred to the 8% brine. In the second method, after filtering the milk through a fabric filter, it was pasteurized at 65°C for 30 min. Then, its temperature was brought to 40°C and the starter with the amount of 1% of milk was added. Milk was left for half an hour for the starter to operate and, after half an hour at 35°C, rennin was added and milk coagulation which took 45 min was completed (rennet was added while the milk was being stirred). The next steps were performed like the process of cheese-making from unpasteurized milk. Immediately after preparing the cheese, a 1 kg sample was taken from every cheese and, after 45 days of maintenance in brine, the second sample was taken. During the three months of fridge maintenance, sampling and analysis procedure were performed every month. Brine maintenance and ripening were done at 10°C and 7±1°C in the fridge, respectively. In fact, for making the cheese using pasteurized milk, brine maintenance operation lasted for 10 days.
Measuring of moisture content of cheese
Moisture content and dry matter of cheese were measured using AOAC 33.7.03 method [10].
Measuring of Total Nitrogen of cheese
Total Nitrogen of the cheese samples was measured using AOAC 99.20 method by an automatic Micro-Kjeldahl device. Total protein was obtained by multiplying the amount of measured nitrogen by conversion factor of 6.38 [10].
Measuring of Non-Casein Nitrogen (WSN)
Kjeldahl approach was used to determine that a 10 g cheese sample could be homogenized by 100 ml distilled water and left for half an hour. After stirring, it was filtered through Whatman filter paper #40 and nitrogen content of the extract was determined using Kjeldahl method [22,10].
Measuring of Non-Protein Nitrogen (NPN)
10 ml of 24% trichloroacetic acid (TCA) solution was added to 10 ml of WSN filtrate and, after stirring for 2 h at 40°C, it was incubated. It was then filtered using Whatman filter paper #40 and Kjeldahl method was used to determine its nitrogen content and expressed as a percentage of total nitrogen. TN/NPN percent was considered an indicator of ripening [22,10].
Determining of Tyrosine concentration using spectrophotometer
First, tyrosine standard curve were prepared. For measuring of the concentration of tyrosine in jug cheese, a 100 g sample of cheese was taken, grated, and then poured into a blender. Then, 50 g of 5.2 % brine was added and blended at low speed for 3 min. 1 g of the prepared mixture was taken, 5.4 ml distilled water was added to it, and was kept in a water bath at 40°C for 5 min. 10 ml of 12% w/v trichloroacetic acid (TCA) was then added to it and remained there until 10 min before filtering using Whatman paper #2. A 5 ml sample of the extract containing TCA was added to 10 ml of a solution containing 15% sodium carbonate and 2% sodium hexametaphosphate maintained at the water bath at 40°C. 3 ml of Folin phenol reagent which was diluted for three times was added to it and the contents of each tube were well stirred. Then, the tubes were left at the 40°C at the water bath for 5 min. Its absorption was measured in the wavelength of 650 nm by a visible spectrophotometer. Concentration of tyrosine was obtained according to the standard curve considering the read absorption rate [16].
Sensory analysis
The ripened cheese samples were evaluated for 30, 60, and 90 days in organoleptic terms by a group of 5 panelists. The samples were randomly given to the panelists and received scores of 1 to 10 in terms of their taste, appearance, and texture.
Statistical analysis
Means of the data obtained from pasteurized and unpasteurized cheese were compared using t-test in MSTATC analysis software.
Results of changes in moisture and nitrogen fractions including Total Nitrogen, Non-Casein Nitrogen, and Non-Protein Nitrogen and Tyrosine concentration during cheese ripening are presented in the following figures:
As is evident in the given diagrams, nitrogen fractions including Total Nitrogen, Non-Casein Nitrogen, Non-Protein Nitrogen, cheese Ripening Indicator, and concentration of tyrosine amino acid increased during the ripening period of both pasteurized and unpasteurized cheese. Statistical analysis of data showed no significant difference between the treatments at two confidence levels of α and α = 5%. Therefore, semi-industrial and hygienic Jug cheese can be made from pasteurized milk. Results of sensory analysis demonstrated that, during the ripening period, taste and flavor of both cheese made of raw and pasteurized milk were improved; but, raw milk cheese had a sharper taste and special smelling, which could be attributed to the presence of coliforms in it according to Kozikowski [15]. Results of this study represented that concentration of Total Nitrogen increased in both pasteurized and unpasteurized cheese during the ripening period and thus protein content in dry matter increased, which might be due to the gradual conversion of fat existing in cheese into volatile fatty acids during cheese ripening [9] and penetration of minerals into whey that increased protein share in dry matter. These results were also observed by Moatsou et al. [8,24,26]. Percentage of Non-Casein Nitrogen or Water Soluble Nitrogen (WSN) in dry matter increased in both types of cheese, which indicated proteolysis during ripening. As a result of proteolysis or decomposition of nitrogenous materials, water insoluble protein materials which are mainly casein turned from insoluble to soluble forms; this result was also obtained in the research conducted by Feeney and Fox on Herby cheese (Figures 2) [6,24,9]. Factors that may cause proteolysis include residual cheese rennet, milk proteinase, mainly plasmin, proteolytic enzymes, starter microorganisms, and proteolytic enzymes in milk flora [1]. In fact, a main part of rennet got out along with whey; but, about 2- 3% of it was still left in cheese and played a proteolytic role during cheese ripening. Plasmin which is a major natural protease of milk is heat-resistant. In pasteurized cheese, its activity increased after milk heating, which could be due to the deactivation of natural milk inhibitors or conversion of plasminogen into active plasmin by heating [17]. It has been proven that coagulant material is responsible for early cheese softening via hydrolysis of αs1-casein in phe24-phe23 bond. Hydrolysis level of αs1-casein depends on the milk coagulating enzyme used in cheese-making. The cheese lacking active coagulant shows no decomposition after ripening, while the cheese with coagulant shows proteolytic changes depending on the used coagulant [2]. In addition to the mentioned factors, proteolysis is influenced by type of coagulant, moisture content of curd, pH of curd, concentration of salt, temperature of ripening, reduction potential, and concentration of cheese minerals [28]. The higher the degree of rennin in the curd, the higher would be the share of αs1-casein hydrolyzed by it (Creamer, 1985). Cow's milk contains numerous proteinase, the most important of which is plasmin that hydrolyzes β-casein to γ-casein (gamma-casein) and other proteose peptones. Proteinases and peptidases also hydrolyze αs1-casein and αs2-casein [19,20]. The amount of proteolysis during cheese ripening is proportional to moisture content. Small changes in casein to moisture ratio change water accessibility, since a large quantity of water is bonded with casein and its decomposition products [22,20]. Change in pH induces effectively changes in the nature of new compounds formed in cheese. Total amount of proteolysis is effectively enhanced at pH of higher than 5.8 [23]. Also, pH is increased at the end of ripening period due to ammonia production (Franco, 2001). Decomposition rate of αs1-casein at lower pH is higher than that of β-casein. Β-casein is decomposed more than αs1-casein at pH of above 5.6, which is probably as a result of increased activity level of plasmin [20]. Percent of salt, salting method, and salt to moisture ratio in curd effectively influence proteolysis speed. An inverse relationship is observed between decomposition rate of both αs1-casein and β-casein and salt to moisture ratio [27]. Higher temperature causes more casein hydrolysis and texture changes. At temperature between 2 and 10°C, texture of cheddar cheese does not change dramatically. αs1-casein is considered a more important structural element in cheese than β-casein and other caseins [5]. Indeed, proteolysis means change of casein fractions into products with less molecular weight. Primary proteolysis in cheese is defined as changes in beta, gamma, and αs1-caseins, peptides, and other smaller bonds which are identified by polyacrylamide gel electrophoresis [1,8,25]. Secondary proteolysis products include changes of peptides, protein, and amino acids dissolved in aqueous phase of cheese that are extractable as water-soluble fractions [22,25]. As shown in the figures, percent of Non-Protein Nitrogen (NPN) in dry matter increased during cheese ripening in both cheese types, which showed proteolysis progress. These findings were in agreement with those by Guinee and Fox on Romano cheese [11,12,8]. The most important Non-Protein Nitrogen compounds include urea, creatine, creatinine, uric acid, erotic acid, hippuric acid, N-peptide, and ammonia which are present with low concentrations in milk and are produced as a result of protein decomposition during cheese ripening. These materials do not precipitate in 12% tri-chloro acetic acid solution along with proteins. Ripening Indicator, determined as a percentage of Non-Protein Nitrogen to Total Nitrogen (as shown in Figure 1) increased during ripening of both cheese types; this result was also obtained during the research by Guinee and Fox on Romano cheese [11]. Such increase was predictable given Non-Protein Nitrogen increase during ripening period. Concentration of tyrosine amino acid, expressed as mg/kg of the sample cheese, also increased during ripening period in both cheese types (as in Figure 5). Concentration of this amino acid was considered the indicator of ripening, which increased with the progress of proteolysis [16,1]. Also, influence of pasteurization on the above factors was studied by t-test at α=1% and α=5% in MSTATC software. Their comparison of means showed no significant difference between the data at these two confidence levels; i.e. Jug cheese, which is a kind of traditional ripened cheese in West Azerbaijan region, can be made of pasteurized milk in order to increase the health level of the product and produce it at industrial scale with more uniform quality. A similar study was conducted by Coskun on Herby cheese which is a type of traditional ripened cheese produced in Turkey that spends its ripening period under soil at 7±1°C, [3].
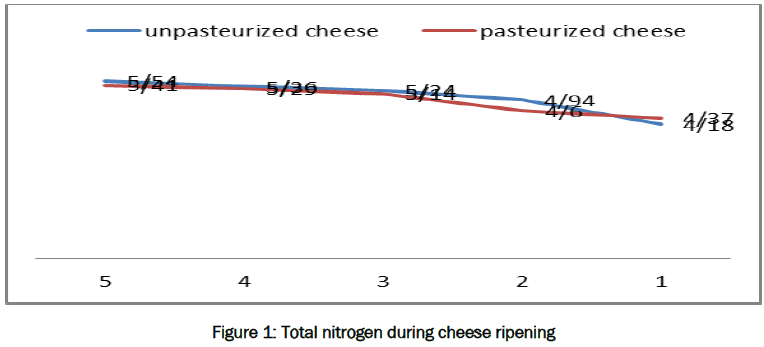
Figure 1: Total nitrogen during cheese ripening
Changes in taste
Scores given to the taste of both cheese during their ripening period increased by the panelists. Flavor, smelling, and texture of cheeses are influenced by the type of used milk (sheep, goat, or cow), complex biochemical reactions during cheese ripening, natural enzymes found in cheese-making milk, applied rennet, starter and nonstarter microorganisms, and proteolysis, lipolysis, and glycolysis reactions that happen under the influence of these factors during cheese ripening [22,8]. Lipolysis and oxidation of fatty acids are the most important reactions in hard cheese types. Nevertheless, proteolysis has the greatest role in the development of flavor and smelling in cheese, which is the result of producing of peptides and free amino acids that make tasty and fragrant compounds such as amines, acids, thiols, and thioesters. Thus, the caseins stored in curd are converted into large and medium peptides by the action of natural plasmin and rennet during cheese ripening and production. Further decomposition occurs by the peptidases of starter lactic acid bacteria released as a result of small peptides and free amino acids. Sulfur containing amino acids, branched and aromatic ones act as precursors for volatile tasty and smelly compounds. It seems that smell and taste of cheese are strongly related to soluble nitrogen compounds, particularly amino acids and small peptides. Fresh cheese has a mild acidic flavor, which takes the characteristics and taste of ripened cheese as a result of ripening. Flavor of ripened cheese is a mixture of different flavoring substances such as diacetyl in mild cheese, butyric acid, caproic acid, ester alcohol, salts of propionic acid and acetic acid in completely ripened cheese, sharp odors of ammonia, and sometimes SH2 in very old cheese [1,7,22].
Since there was no statistically significant difference between ripening factors of the cheese made of pasteurized and non-pasteurized milk, pot cheese, as a kind of traditional Iranian cheese, can be produced from pasteurized milk at an industrial scale in order to provide the possibility of healthy cheese production at a large scale. Moreover, maintenance period of fresh cheese in brine can be reduced to 10 days, during which uniform salt penetration into the cheese occurs, because brucellosis-causing pathogenic bacteria are removed in the initial pasteurization process of milk and it is not necessary to maintain the initial cheese in 8% brine for 45 days. Thus, cheese preparation period is also reduced after milk pasteurization.
This study was financially supported by Urmia University under the cooperation of respected professors and staff of Department of Food Science and Technology, Faculty of Agriculture, Urmia University.