ISSN ONLINE(2278-8875) PRINT (2320-3765)
ISSN ONLINE(2278-8875) PRINT (2320-3765)
Lieutenant. J. Ganesan1, D. Edison Selvaraj2 and J.Ramathilagam3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Advanced Research in Electrical, Electronics and Instrumentation Engineering
Thermal conductivity plays an important role on the thermal withstand capability of the insulating materials. It has been observed that the use of nano composites in the matrix of polymeric materials can greatly improve the thermal, mechanical and electrical properties of polymeric nano composites. A nano composite (TiO2 +SiO2) has been tested as nano filler. The micro particles of TiO2 and SiO2 are converted into nano particles with the help of ball mill. Scanning electron microscope (SEM) has been used to augment the particle size of nano composite. These nano composites were mixed with standard (Elmo Luft 1A-FD) enamel with help of ultrasonic vibrator. The thermal conductivity of enamel, micro composite (3:1, 1:3, 1:1 of SiO2 and TiO2) filled enamel and nano composite (3:1, 1:3, 1:1 of SiO2 and TiO2) filled enamel were detailed and analysed. Thermal conductivity was measured by using Lee’s Disc method. The mixing of nano composites of SiO2 and TiO2 with enamel in various proportions has no improvement when compared to the thermal conductivity of the enamel and the enamel filled with micro composites of SiO2 and TiO2 but reduced thermal conductivity when compared to that of SiO2 and TiO2. The enamel and micro composite of SiO2 and TiO2 taken in the proportion of 3:1 filled enamel has same value of thermal conductivity.
Keywords |
| Thermal Conductivity, Ball Mill, SiỎ̢̉, TiỎ̢̉, Scanning electron microscopy. |
INTRODUCTION |
| Thermal conductivity was the property of a material describing its ability to conduct heat. Thermal conductivity was measured in W·K-1·m-1. Enamel has higher thermal withstand capability. In the last few years, a great deal of attention has been given to the applications of nano dielectrics in the field of electrical insulating materials. It has been reported that the use of nano composites in the matrix of polymeric materials can greatly improve the thermal, mechanical and electrical properties of polymeric nano composites [5].In the last few years, a great deal of attention has been given to the application of nano dielectrics in the field of electrical materials [3]. Nano dielectrics were a class of materials containing at least one phase at the nano meter scale. It has been reported that the use of nano fillers improves the corona resistance of polyimide films [2]. In addition, the use of nano composites of Silicon Di-Oxide (SiO2) and Titanium oxide (TiO2) in low density polyethylene has shown a smaller decrease in the resistivity of the polymeric matrix compared to micro fillers. A driving force of the nano revolution was a continuous progress in the nano dielectrics towards increasing the level of stability, the reduction in the size and weight of the insulating materials [6]. Various studies have been made by comparing the performance of nano and micro particle filled enamel. In this paper, the thermal conductivity of enamel, micro composite (3:1, 1:3, 1:1 of SiO2 and TiO2) filled enamel and nano composite (3:1, 1:3, 1:1 of SiO2and TiO2) filled enamel were analysed. |
SYNTHESISATION AND CHARACTERIZATION OF NANO PARTICLES |
| A. Ball Mill Method |
| There were several methods for creating nano particles, including both attrition and pyrolysis. In attrition, macro or micro scale particles were ground in a ball mill, a planetary ball mill, or other size reducing mechanism. Ball mill was an efficient tool for converting micro powder into nano powder [5]. There are two ways of grinding: dry process and the wet process. The Silicon dioxide and titanium oxide nano filler was synthesized by this method. The pulverization planetary ball mill as shown in figure 1 was universally applicable for quick dry or wet grinding of inorganic and organic samples. The sample material was crushed and disintegrated in a grinding bowl by grinding balls. The grinding balls and the micro powder in the grinding bowl were acted upon by the centrifugal forces due to the rotation of the grinding bowl about its own axis and due to the rotating supporting disc. |
 |
| B. Characterization Of SiO2 And TiO2 |
| Nano particle characterization was necessary to establish understanding and control of nano particle synthesis and applications. Characterization was done by using a variety of different techniques such as transverse and scanning electron microscopy (TEM and SEM), atomic force microscopy (AFM), dynamic light scattering (DLS), x ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), ultraviolet-visible spectroscopy and nuclear magnetic resonance (NMR). Hitachi SU1510, shown in figure 2 was a compact, high performance scanning electron microscope. It was used for analysing the particle size of SiO2and TiO2 micro and nano particles. High resolution imaging was provided by this electron microscope. The SiO2and TiO2 particles were subjected to scanning electron microscopy to analyse the particle size and structure of the nano particles. |
 |
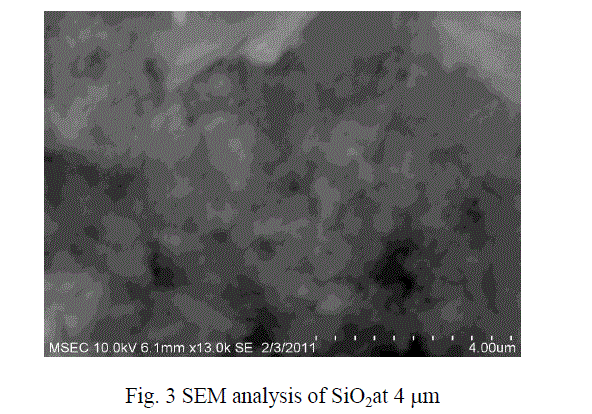
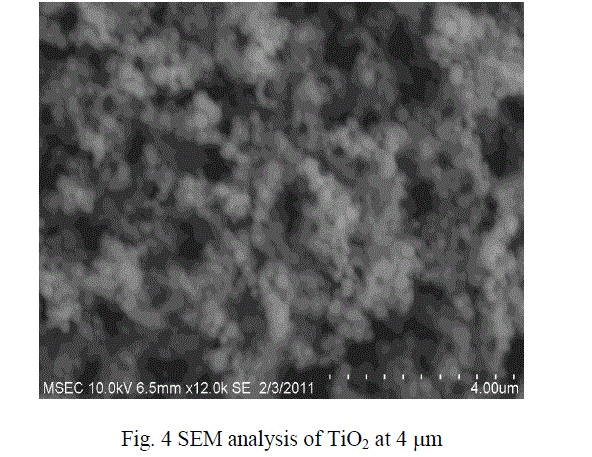
| The micro size particle are converted into nano size with the help of Ball Mill .The SEM results show that the particles were in the form of nano metric range varies. The sizes of the particles were in the range from 40 to 100 nm size. The figures 3 and 4 show the SEM analysis of SiO2 and TiO2 after ball mill synthesisation method. |
 |
 |
| C. Curing |
| The process of converting liquid state enamel into solid state sample is called as curing. The curing method used for enamel was radical initiator curing. In this process DDM (Diamino Diphenyl Methane) was used as curing agent [1]. 80% of enamel and 20% of epoxy resin was taken. DDM was taken in proportion to epoxy resin. For 1g of resin, 0.27 g of DDM was added. DDM was melted for 10 minutes for 60-80º C. The enamel, resin and melted DDM were mixed in a beaker. The mixture was poured in the die which is coated by a Teflon sheet. Then the die was heated at 120º C for 2 hours and 130º C for 3 hours in an oven. The die was cooled and the solid sample was taken away from the oven. |
EXPERIMENTAL RESULTS |
| A. Lee’s Disc Method |
| The thermal conductivity of the different samples was measured by Lee’s disc method [7]. Lee’ disc, Thermometers, steam generator, Screw Gauge and Vernier caliper were used for this method. The mean thickness of sample and the thickness of the disc were determined by Screw Gauge. The diameter of the disc was measured by using Vernier Caliper. The mass of the disc was found by spring balance. The thickness of the sample and Lee’s disc were 3 mm. The radius of the disc was found as 5.52 cm. The mass of the disc was 0.79 kg. The given sample was placed between the disc and steam chamber. The two thermometers were inserted into the radial holes drilled on the side of the metallic discs. Steam was then passed through the steam chamber from a boiler until the steady state condition. The steady temperatures of the disc and steam chamber were recorded by the thermometers. The sample was then removed and the disc was heated directly until the temperature of the disc rises by about 5°C about θ1. Then the steam chamber was removed, and the disc was allowed to cool. The stop clock was started and the time for every 1°C fall in temperature of the disc was noted until the temperature of the disc falls 5°C below θ1. The cooling curve was drawn with Time along X-axis and the Temperature along the Y-axis. The rate of cooling dθ/dt at θ1 was determined. The value of thermal conductivity of the various samples were found using the formula |
| K=MS * dθ/dt * [(r+2l)/ (2r+2l)] * [d/ πr2] * 1/ [θ2-θ1] W m-1 K-1 |
| Where |
| • M =Mass of the disc in Kg |
| • S =Specific heat of the material of the disc in J-1Kg-1K |
| • r = Radius of the disc in meter. |
| • l = Thickness of disc in meter. |
| • d = Thickness of the sample |
| • θ1 = Steady temperature of the disc in K. |
| • θ2 = Steady temperature of the steam chamber in K. |
| • dθ/dt =rate of cooling. |
| The thermal conductivity of enamel, micro composite (3:1, 1:3, 1:1 of SiO2 and TiO2) filled enamel and nano composite (3:1, 1:3, 1:1 of SiO2 and TiO2) filled enamel were shown in the table 1. |
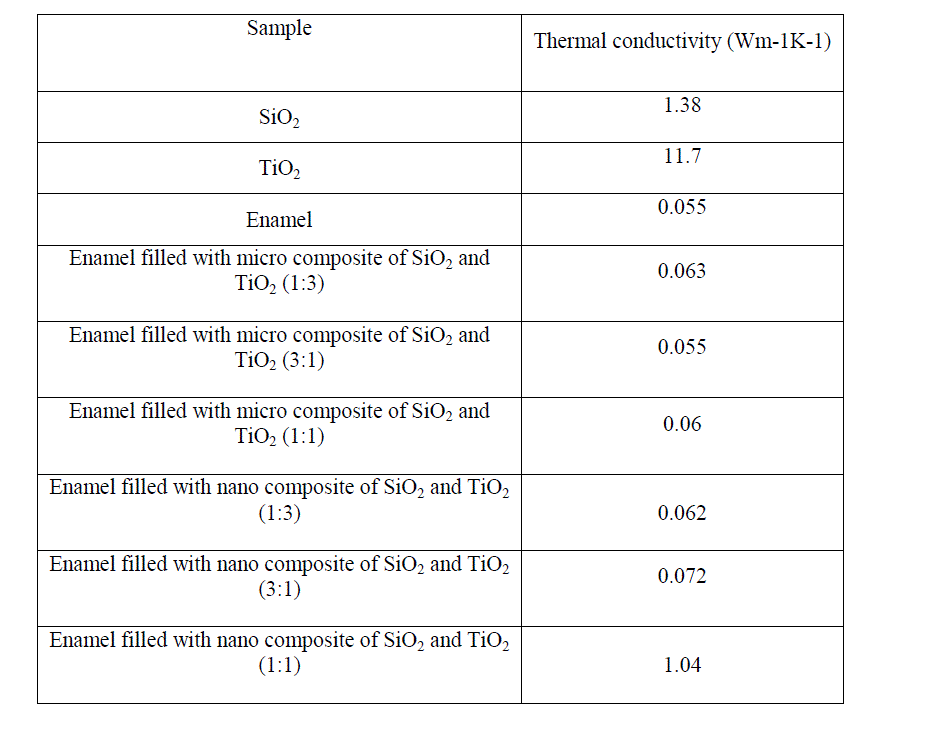 |
| The mixing of nano composites of SiO2 and TiO2 with enamel in various proportions has no improvement when compared to the thermal conductivity of the enamel and the enamel filled with micro composites of SiO2 and TiO2 but reduced thermal conductivity when compared to that of SiO2 and TiO2. The enamel and micro composite of SiO2 and TiO2 taken in the proportion of 3:1 filled enamel has same value of thermal conductivity. |
CONCLUSION |
| SiO2 was having low thermal conductivity and TiO2 was having higher thermal conductivity. The addition of micro and nano composite of SiO2 and TiO2 to the enamel has no significant impact on the thermal conductivity of the enamel. It has been found the thermal withstand capacity of the enamel does not changes with the addition of micro and nano composite of SiO2 and TiO2. When the enamel was mixed with micro and nano composite of SiO2 and TiO2 in various proportions, the thermal conductivity of overall composite was reduced to a considerable value. There was only a slight modification in the value of thermal conductivity of the enamel. The enamel itself was having lower thermal conductivity when compared to SiO2 and TiO2. The lower value of thermal conductivity implies that the enamel has good insulating property The mixing of nano composites of SiO2 and TiO2 with enamel in various proportions has no improvement when compared to the thermal conductivity of the enamel and the enamel filled with micro composites of SiO2 and TiO2 but reduced thermal conductivity when compared to that of SiO2 and TiO2. The enamel and micro composite of SiO2 and TiO2 taken in the proportion of 3:1 filled enamel has same value of thermal conductivity. |
ACKNOWLEDGEMENT |
| Thank God and His almighty power to finish His research work by using me, and my friend for His ultimate work. |
References |
|