ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
G.V.Pandian1, P .Anbusrinivasan2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Thiosemicarbazone of Benzophenone(TSCBP) is a semi-organic crystal which is grown by solution growth technique by adopting slow evaporation method from the solvent methanol. The crystal dimension up to 14X6.5X4mm3 obtained. The harvested crystal were purified by repeated recrystallization .The induction period was measured at various super saturations and hence the interfacial energies were evaluated. Using the interfacial tension value, the nucleation parameters such as radius of the critical nuclei(r*), the Gibbs free energy for the formation of a critical nucleus (ΔG*) and the number of molecules in the critical nucleus(i*) were also calculated for various concentration of solvent at two different temperatures. The effect of surface tension, viscosity and density of these solvents are correlated with interfacial tension. These crystals were characterized by FT-IR spectra to identify the functional group present in the compound. The optical transparency was examined by UV-Spectral analysis. The molecular structure was analyzed by chemical environment of magnetic nuclei such as 1H and13C. The TGA and DSC confirm the decomposition of the sample at 184.140c. It confirms the grown crystal Thiosemicarbazone of (TSCBP) is thermally stable up to 184.140c .The grown crystal was examined by X-ray diffraction to determine its crystalline nature. Second harmonic generation efficiency of the powdered Thiosemicarbazone of benzophenone (TSCBP) was tested using Nd:YAG laser and it is found to be ~0.8 times that of potassium dihydrogen phosphate
Keywords |
| Solution growth, slow evaporation technique, Induction period, Interfacial energy , spectral characterization, Thermal analysis, SHG efficiency |
INTRODUCTION |
| For the past three decades,the field of opto electronics,optic communication have experienced tremendous advancements .Recently the Nonlinear material are gain more attention due to their enormous application in opto electronic and optic communication technologies[1-2]. Organic compounds are often formed by very weak Vander walls and hydrogen bonds and possess high degree of delocalization .Hence they are optically more nonlinear than inorganic crystals. Generally .Recent researches have mentioned that organic crystals are bulk in size,hard,stable, and large Nonlinear optical susceptibilities compared to the inorganic crystals but they have poor mechanical properties .Considering all these parameters the modern scientists have concentrated on the growth of semi-organic crystals .The semi-organic crystals of Thiosemicarbazone crystals have high thermal stability and Non-linear optical properties, In addition Thiosemicarbazone molecules containing π-electron conjugation system asymmetrized by the electron donor and acceptor groups are highly polarizable entities for NLO applications. Hence the Thiosemicarbazone of benzophenone is the one of the potential semi-organic optical materials. Which belongs to the carbonyl group of compounds .The Ketone group is asymmetrical (Chiral carbon). Therefore in the present studies We report on the preparation Growth,Spectral,Thermal,Induction period, Interfacial energy and Nucleation parameters of non-linear optical Thiosemicarbazone of benzophenone (TSCBP) crystals by slow evaporation solution growth technique(SESGT).Metastable zone width is an essential parameter for the growth of good crystals from solution. Because it is the direct measure of the stability of the solution in its saturated region. The growth and the metastable zone width of Thiosemicarbazone of benzophenone (TSCBP) in pure methanol, 1:1 methanol, 1:2 methanols as solvent were determined. The harvested crystals were subjected to FT-IR, UV, 1H, 13C NMR, XRD, TGA-DSC analysis and NLO studies for characterization [4].The nucleation parameters solution grown Thiosemicarbazone of benzophenone (TSCBP) were determined using the interfacial tension and reported for the first time. |
EXPERIMENTAL |
| Thiosemicarbazone of benzophenone (TSCBP) was prepared by reacting analytical grade Thiosemicarbazide and Benzophenone in methanol,All chemicals used in this study were supplied by E.Merk.Aldrich Chemical Co.IR Spectra were recorded in a AVTAR 370 DTGS FT-IR spectrometer using KBr pellets.UV Spectra were recorded using a Lambda 25 UV-Visible spectrometer.C13 and 1H NMR spectra were recorded in BRUKER NM-474. Thermal studies have been carried out using on SDTQ 600R 20.9 BUILD 20 Instrument. X-ray diffraction patterns of the samples were recorded on a BRUKER D8 ADVANCE POWDER diffractometer with Cu Kα radiation. |
| The semi-organic crystal of Thiosemicarbazone of benzophenone is prepared by adopting the standard procedure [3]. To a hot solution of Thiosemicarbazone in methanol, a solution of benzophenone in methanol was added drop wise during 30 minutes. The mixture was stirred and refluxed for four hours .Then it was filtered and the filtrate was concentrated to half the volume.Then the filtrate allowed for slow evaporation at room temperature, crystals were collected by filtration, washed with cold ethanol and dry the crystals in vacuo.The harvested crystals are shown in figure 1.These crystals are suitable for characterization studies. |
 |
RESULT AND DISCUSSION |
| A. FT-IR SPECTRAL ANALYSIS |
| The Infra-red spectral analysis has been carried out to understand the chemical bonding and it provides useful information regarding to the molecular structure of the compound [4].The spectrum was recorded using AVTAR 370 DTGS FT-IR spectrometer in the wave number range from 400 cm-1 to 4000cm-1. With KBr pellet technique. The Fourier transform infrared spectrum of Thiosemicarbazone of benzophenone is shown in figure 2.The observed and their corresponding group identification is given in Table 1. The peak at 3365cm-1 shows the C-H stretching vibration of alkynes .The peak at 1482.02cm-1 shows N-H and S-N stretching vibration[5].The peak at 1160.43cm-1 shows C=S stretching vibration.The peak at 1278 cm-1 corresponds to aromatic C-H. The peak at 999.89cm-1 indicates C-N stretching. The peak at 647cm-1 shows N-H bending of 20amines. |
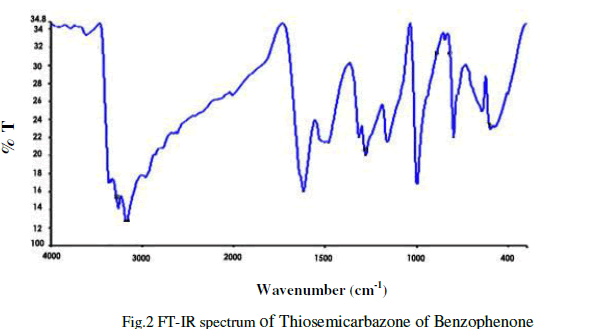 |
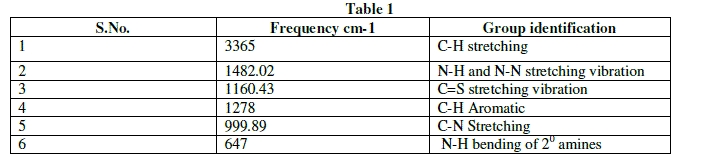 |
| B. UV-VISIBLE SPECTRAL STUDIES |
| UV-Visible Spectral study is very useful technique to determine the optical properties and transparency of a substance[6].The UV-Visible spectrum of Thiosemicarbazone of benzophenone (TSCBP) crystals was recorded using a Lambda 25UV-Visible spectrometer is shown in figure 3. |
| The spectrum shows the characteristic absorbance band is 310.46 nm. The UV cut off of Thiosemicarbazone of benzophenone (TSCBP) is 310.46nm and there is no absorbance band between 325-950 nm. Because of these properties Thiosemicarbazone of benzophenone (TSCBP) is useful for optoelectronics applications .Optical properties of crystalline materials give information regarding the composition nature and quality of the crystal. |
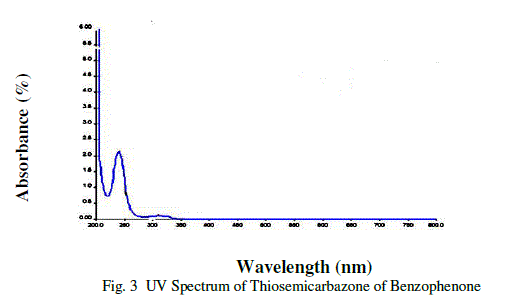 |
| C. NMR SPECTRAL ANALYSIS |
| Based on the chemical environment of the magnetic nuclei like 1H, C13, the NMR Spectral studies is used to determine molecular structure[7] .The 1H NMR spectral analysis was carried out on the Thiosemicarbazone of benzophenone(TSCBP) crystal in BRUKER NM-474.The 1H NMR spectra of Thiosemicarbazone of benzophenone (TSCBP)is shown in figure10 The observed and their corresponding group identification are given in Table 2.The 1H NMR spectrum revealed an aromatic system at δ= 7.721ppm. (There is a multiplet at δ=7.721 ppm indicates the presence of aromatic group.The NH2 proportion of hydrazide is observed at 8.658 as broad singlet .The hetero atom sulphur observed at 7-8 ppm with 1.29Hz. The –NH proton is observed at 7.219 ppm, Because of the results the presence of hydazide group which confirmed by NMR spectral analysis. |
 |
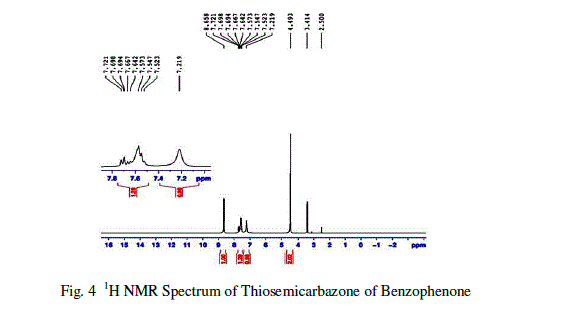 |
| 13C NMR SPECTRAL ANALYSIS |
| In the 13C NMR spectral studies also used to determine the molecular structure of the compound [8]. The singlet observed at δ=196.03 ppm shows the presence of non-chelated ketone carbon. The peak observed at δ= 137.04ppm confirms the sp2 hybridized carbon atom. The singlet at δ=132.88 methine sp2 hybridized carbon atom. The methoxy carbon proves the peak observed at δ= 129.73ppm and δ= 128.73 ppm.The multiplet observed at δ =40.36 ppm shows the presence of methyl group and sulphur atom. |
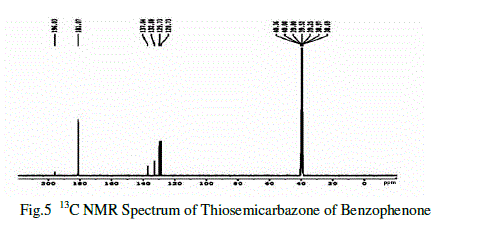 |
| D. THERMAL ANALYSIS |
| Thermal properties of harvested crystal of Thiosemicarbazone of benzophenone were studied in powder form by recording TGA and DSC response curve in the temperature range between 00C to 5000C. Thermal studies have been carried out using on SDTQ 600R 20.9 BUILD 20Instrument at a heating rate of 10 0c/min under nitrogen atmosphere. The thermograme of Thiosemicarbazone of benzophenone shows in figure 6.The sample taken for the measurement is 16.6580 mg. There is an endothermic peak at 184.14 0c shows its melting point. The thermogram further shows the thermal stability and crystalline nature of the grown crystal [9].Because of the sharp endothermic peaks shows the good degree of crystallinitys of the Thiosemicrbazone of benzophenone. The other endothermic peak shows the further decomposition .In TGA there is a sharp weight loss at 206.750c and further the curve shows subsequent weight loss at 246.09 0c and 283.930c. |
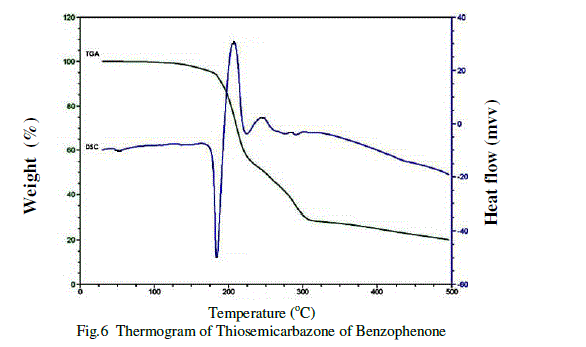 |
| E. X-RAY DIFFRACTION STUDIES |
| The recorded X-ray diffraction spectrum for Thiosemicarbazone of benzophenone sample is shown in the figure.7.X-ray diffraction patterns of the samples were recorded on a BRUKER D8 ADVANCE POWDER diffractometer with Cu Kα radiation (α=1.5418A0) scanned rate of 1o/min in the range 10-700c.The crystalline nature and purity of the grown crystals determined by X-ray diffraction studies [10].The experimental values of 2θ and d were obtained from the powder XRD spectrum. The obtained values are in good agreement with literature values and also confirmed that the grown crystal is Thiosemicarbazone of benzophenone. |
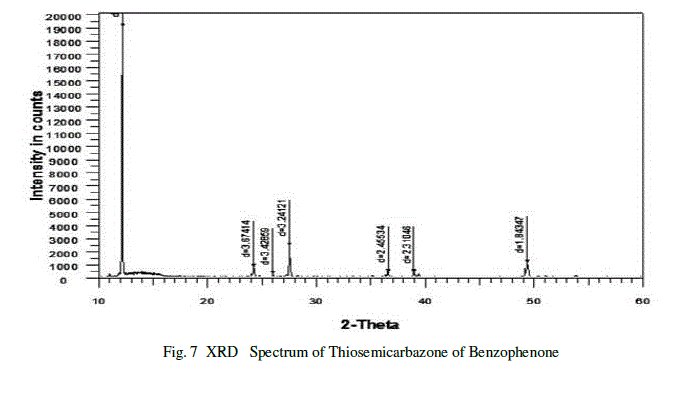 |
| F. DETERMINATION OF METASTABLE ZONE WIDTH |
| Metastable zone width is an important parameter for the growth of good crystals from slow evaporation solution growth technique. It is very useful direct measure of the stability of the solution in the supersaturated region[11]. The metastable zonewidth of Thiosemicarbazone of benzophenone in 1:1, methanol,1:2 methanols were determined. Saturated solution of Thiosemicarbazone of benzophenone in these solvent ratio at different temperature s were allowed for systematic slow cooling .The temperature at which the first nucleation was observed which corresponding to their width of the metastable zones.The metastable zonewidth of Thiosemicarbazone of benzophenone in methanol shown in figure ,Blue line indicates metastable zone width and red line indicates solubility. |
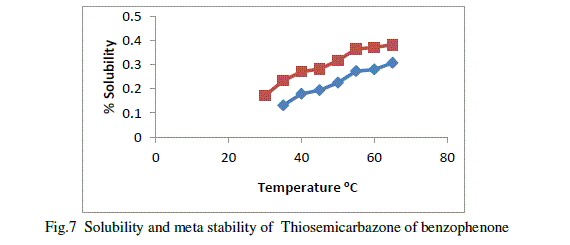 |
| G. INDUCTION PERIODS AND INTERFACIAL ENERGIES |
| There are several methods of measuring the induction period depending upon the solubility of the materials. Here the visual observation method was followed.Solutions of Thiosemicarbazone of benzophenone in methanol at different supersaturation values were prepared and subjected to systematic slow evaporation.The time period that elapses between the achievement of supersaturation and appearance of visual nuclei is taken as the induction period (t). Several trial runs were performed to minimize the error. Experiments were repeated for supersaturation (s) like 1.15, 1.17, 1.20 and 1.25at two different temperatures. From the results obtained a plot of lnt against 1/(lns)2 is drawn and is shown in figure11.The interfacial tension was calculated from the slope of the curves using the equation |
| lnt =lnA+16πr3V2N/3RT(ln s)2 |
| Where A is a constant related to the pre-exponential factor of the nucleation rate expression. V is the molar volume, N is the Avogadro number and R is the gas constant. The factor 16πr3 in the above equation refers to the spherical nuclei. The interfacial tension between the TSCBP and methanol is calculated by measuring the slope value of the curve obtained at the two temperatures. |
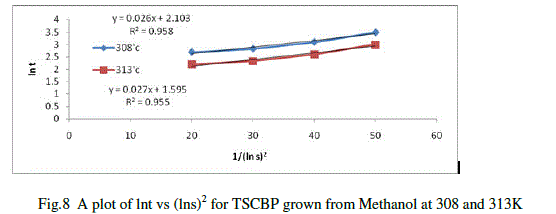 |
| H. NONLINEAR OPTICAL STUDIES |
| Kurts and perry second harmonic generation (SHG) tests [12]was performed to determine theNLO efficiency of Thiosemicarbazone of benzophenone (TSCBP) crystal.The grown crystal was powdered with a uniform particle size and packed in a micro capillary of uniform bore and was illuminated using spectra physics quanta ray DHS2.Nd:YAG laser is used to test second harmonic generation (SHG) of grown crystal,The SHG efficiency obtained for Thiosemicarbazone of benzophenone (TSCBP) is about 0.8 times that of potassium dihydrogen orthophosphate crystal. |
CONCLUSION |
| The meta stable zonewidth of various concentration of methanol were determined for the first time it was found that the meta stable width dependence on solvent concentrations.The effect of temperature and solvent on interfacial tension was determined using the interfacial tension value the nucleation parameters such as radius of the critical nuclei (r*) , the Gibbs free energy for the formation of a critical nucleus (ΔG*) and the number of molecules in the critical nucleus(i*) were also calculated for various concentration of solvent at two different temperatures. The effect of surface tension, viscosity and density of these solvents are correlated with interfacial tension optically good grade of semi-organic Thiosemicarbazone of benzophenone crystal were successfully grown using slow evaporation solution growth technique, using benzophenone and Thiosemicarbazide using methanol as a solvent. The methanol grown crystals are good quality . The FT-IR spectral report confirms the purity and functional group of the crystal. The UV-Visible spectrum proves the transparent nature of the crystal between 325-950nm.The 1H and13CNMR spectral analysis also characterize the molecular structure[37,38] .The TGA and DSC traces confirm that the crystal is thermally stable at 184.140C.The grown crystal of Thiosemicarbazone of benzophenone was confirmed by X-ray diffraction analysis. The optical transparency, thermal stability, and crystalline nature of Thiosemicarbazone of benzophenone confirm its various applications. |
ACKNOWLEDGEMENT |
| The authors wish to thank Dr.J.Samu Solomon, Department of Chemistry,TBML College |
References |
|