ISSN:2321-6212
ISSN:2321-6212
Chang Zhijuan1,2, Wang Qiangwei1, Hou Jiawen4*, Wu Xuehong1,3*, Lv Cai1,3, Liu Yong1,3
1 Zhengzhou University of Light Industry, Zhengzhou 450002, China.
2 Key Laboratory of Cold Chain Food Processing and Safety Control (Zhengzhou University of Light Industry), Ministry of Education, China, 450002.
3 Henan International Joint Laboratory of Energy Efficient Conversion and Utilization, Zhengzhou, Henan, China, 450002.
4 Zhumadian Cigarette factory, China Tobacco Henan Industrial Co., Ltd., Zhumadian 463002, China.
Received: 27-May-2024, Manuscript No. JOMS-24-137362; Editor assigned: 31- May -2024, PreQC No. JOMS-24-137362 (PQ); Reviewed: 14-Jun-2024, QC No. JOMS-24-137362; Revised: 21-Jun-2024, Manuscript No. JOMS-24-137362 (R); Published: 28-Jun-2024, DOI: 10.4172/2321-6212.12.2.001
Citation: Zhijuan C, et al. Experimental Study on the Optical Properties and Photothermal Conversion of Biomass Composite Phase Change Materials. RRJ Mater Sci. 2024;12:001.
Copyright: © 2024 Zhijuan C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Research & Reviews: Journal of Material Sciences
Solar energy is a renewable source of energy. The advantages of solar energy include its wide distribution, short cycle, high power, easy availability, and no pollution. As solar energy is seriously affected by changing weather, large-scale utilization of solar energy is restricted. Organic Phase Change Materials (PCMs) are an ideal thermal energy storage medium, and the development of solar-thermal energy conversion technology requires materials to effectively capture and store solar energy. However, PCMs have some characteristics that need improvement, such as low light-to-heat conversion rates. Therefore, this article added Biomass Porous Carbon (BPC) to improve the thermal conductivity and optical properties of PCMs. The experimentally determined thermal conductivity of 70% Paraffin Wax (PA)-(BPC) Composite PCM (CPCM) was 3.18 times higher than that of pure PA. In addition, the average absorbance in the range of 190–2000 nm was approximately 1.3, and approximately 95% of solar radiation was absorbed by the CPCM and stored as thermal energy. The photo thermal conversion efficiency of the CPCM was as high as 89.6%, thus infrared thermal image analysis was used to discover the positive effects of biomass porous materials on solar light capture and heat transfer. Therefore, composite BPC-based PCMs have broad application prospects in light-to-heat conversion and energy storage.
Composite Phase Change Material; Energy Storage; Solar Energy; Absorbance; Light-To-Heat Conversion Rate.
The demand for renewable, sustainable energy is rising because of global efforts to reduce fossil fuel use and carbon emissions [1,2]. Among renewable energy sources, solar energy is a green pollution-free energy source, which has the advantages of wide distribution and easy availability [3]. However, the use of solar energy is affected by weather (cloudy or rainy) and time (dark night), and solar photo thermal conversion technology effectively solves this problem. Solar photo thermal conversion technology converts sunlight into heat energy and stores it [4]. The conversion efficiency of solar energy to thermal energy can reach more than 80%; this method is the future of solar energy utilization [5]. The key to solar-photo thermal conversion technology is to find materials that have a high energy storage density and can effectively absorb and convert sunlight into heat over time.
Organic Phase Change Materials (PCMs) exhibit good chemical properties and high energy storage density, and the temperature does not change significantly when storing heat energy. Therefore, these materials have good application prospects in energy storage [6]. Although organic PCMs can be used as energy storage materials in solar photo thermal conversion technology, traditional organic PCMs have inherent shortcomings: Their thermal conductivity is low, and their ability to absorb sunlight and convert light to heat is poor, which affects the utilization efficiency of solar energy [7-9]. These shortcomings seriously affect the application of organic PCMs in solar photo thermal conversion and storage technologies.
In view of the defects of Composite PCMs (CPCMs) in solar photo thermal conversion and storage technologies, an increasing number of studies have focused on adding materials with good optical properties and high thermal conductivity to PCMs. The prepared CPCMs not only have improved photo thermal conversion performance but also improved heat transfer rates when charging and discharging heat. In this context, an increasing number of researchers have used this method to make CPCMs more widely used among solar-thermal conversion and storage technologies [10]. Used melamine sponge as a support material, Paraffin Wax (PA) as a solid-liquid PCM, and reduced graphene oxide and zirconium carbide as additives to enhance heat conduction and solar light absorption, and developed a new PCM with a stable morphology. Those zirconium carbide CPCMs with different contents have good light absorption, high heat storage capacity, and excellent heat transfer performance; in addition, the light-to-heat conversion efficiency can reach 81% [11]. Used solid sodium acetate as a raw material to prepare a graphene framework with large specific surface area, high thermal stability, and high thermal conductivity through a pyrolysis method, and the photo thermal conversion rate of the prepared composite phase change material is an increase of approximately 78% [12]. combined experimental and numerical methods to study the optical properties of PA doped with ZnO or CuO nanoparticles, and analyzed the influence of nanoparticles on the thermal and optical properties of PA. The results show that because of the presence of nanoparticles, the light transmittance of nano reinforced PA is reduced, and the light absorption rate is increased. The volume fraction of metal particles has the best light and heat performance in the range of 5×10−4–1.5×10−3% [1]. Prepared high-quality graphene aerogels under high-temperature conditions and CPCMs via vacuum impregnation. The CPCMs have excellent light-to-heat conversion capabilities, with a conversion efficiency of up to 84% [13]. introduced functionalized graphene nanosheets to prepare new CPCMs. Those materials not only effectively prevent the leakage of the PCM, but also improves the thermal conductivity, and the CPCM has a higher phase change enthalpy (248.3 J/g). The addition of graphene nanosheets significantly improves the solar light absorption characteristics of the PCM and provides a high light-heat efficiency of 92.6% [14]. used a one-pot method to synthesize a light-driven polymer composite with high thermal conductivity and phase change enthalpy. The latent heat value of the composite material is 180.3 J/g, and the light-to-heat conversion efficiency is 72.1% [15]. Prepared a new type of stable CPCM using a vacuum impregnation method. The results show that the material has good phase change performance, low sub cooling, good thermal cycle stability, and good solar light absorption performance; in addition, the enthalpy of fusion is 207.3 J/g, and a light-to-heat conversion efficiency of up to 80.6% is achieved [16]. Used sucrose and sodium bicarbonate as raw materials to prepare a CPCM with excellent performance using a template method. The thermal conductivity of the CPCM was increased by 180% and the light-to-heat conversion efficiency was as high as 89%.
At present, when PCMs are applied to solar-thermal energy conversion technology, there are still problems such as low light-to-heat conversion rates. Previous work has verified that Biomass Porous Carbon (BPC) and its CPCMs have high thermal conductivity and good leakage resistance. In this study, the optical properties of BPC and its CPCMs, as well as the photo thermal conversion ability of CPCMs under simulated sunlight, were investigated. The results confirmed that the bio-made porous carbon proposed in this study had good light-to-heat conversion performance. Approximately 95% of solar radiation was absorbed by the CPCM and used in thermal energy storage, and a light-to-heat conversion efficiency of 89.6% was obtained. The CPCM designed and synthesized in this study has a wide range of applications in the field of solar thermal energy conversion and storage.
Experimental
PA, with a phase transition temperature of 40°C, was purchased from Hangzhou Ruler Energy Technology Co., Ltd. (Hangzhou, China). An acrylic sample mold with an inner diameter of 15 mm, height of 30 mm, and thickness of 2 mm was purchased from the Tengxiang Plexiglass Factory. Analytical pure anhydrous ethanol and hydrochloric acid, 98% concentration, were purchased from Zhengzhou Liyan Technology Co. Ltd.
Preparation of biomass carbon materials
Sawdust was soaked in hydrochloric acid for 12 hrs, followed by soaking and washing with deionized water for 12 hrs. To remove soluble impurities and surface inorganic matter, the washed sawdust was placed in a drying oven at 80°C until the material quality did not change. The dried wood chips were placed in a high-temperature furnace; the temperature was set to 900°C, and the heating rate was set to 5°C/min, passed in N2 gas, and carbonized for 3 h. Then, the obtained sawdust charcoal was ground into a powder to obtain BPC.
Characterization
First, PA was heated to 85°C until it melted completely. The melted PA was then mechanically stirred and ultra-sonicated in a glass container according to different mass fractions of PA and porous carbon for 2 hrs. Finally, the mixture was uniformly mixed. The CPCM was placed in a customized mold and pressed into a PCM block with the same quality and thickness. An external visible and near-infrared spectrophotometer (UV-3600 Plus, Shimadzu, Kyoto, Japan) was used to measure the transmission spectrum, reflection spectrum, and absorption spectrum of PA and the CPCMs, with a wavelength accuracy of ± 0.1 nm. Samples of the same weight and thickness were placed on a thermal insulation platform and exposed to a solar simulator (7IS1003A, Sofn Instruments Co., Beijing, China) under a xenon lamp source. The intensity of the light source was 164 W/m2, and the accuracy of the light source intensity was ± 0.1 W/m2. The acquisition instrument (temperature accuracy of 0.1°C, Agilent Technologies, Santa Clara, CA, USA) recorded the temperature as a function of time and calculated the light-to-heat conversion rate of the CPCM.
Thermal analysis
Most organic PCMs have low thermal conductivity, which reduces the energy storage and release rate, thus limiting the practical application of these materials. The addition of high-thermal conductivity materials can effectively improve the thermal conductivity of organic PCMs. The thermal conductivities of pure PA and porous CPCMs are shown in Figure 1. As shown in Figure 1, as the PA content decreased, the thermal conductivity continued to increase. The thermal conductivities of pure PA, 90% PA-BPC, 80% PA-BPC and 70% PA-BPC were 0.240, 0.497, 0.606, and 0.763 W/m·K, respectively. The thermal conductivities of the porous composite PCMs were 2.07–3.18 times higher than that of pure PA (0.24 W/m·K). Carbonization and cross-linking reactions adjust the structure of porous carbon and promote graphitization of three-dimensional porous carbon networks, thereby increasing the average speed and mean free path of phonons [17]. Therefore, under the synergistic effect of high-temperature carbonization and crosslinking reactions, the strong thermal vibration of phonons greatly improved the thermal conductivity of the CPCM. From a macro point of view, the powder had a larger specific surface area. Compared with porous carbon carbonized into a block, the contact area with the PCM was larger and exhibited better thermal conductivity.
Energy storage characteristics
The latent heat value of the phase change is an important parameter for evaluating the energy-storage capacity of CPCMs. Figure 2 (a) and (b) show the Differential Scanning Calorimetry (DSC) temperature rise and fall curves of pure PA and the prepared composite material with different porous carbon contents. The melting and crystallization temperatures of pure PA were 41.19°C and 42.51°C, respectively, and the melting and crystallization temperatures of the CPCM (70% wt PA) were 39.25°C and 41.43°C, respectively. The decrease in the melting temperature might be due to the addition of carbon-based materials to improve the thermal conductivity of the composite material. When the temperature increased, the melting of PA accelerated.
The latent heat of melting and crystallization of the PCM were obtained by calculating the heating and cooling heat flow areas in the DSC curve. As shown in Table 1, the melting and crystallization enthalpies of PA were 212.3 kJ/kg and 226.4 kJ/kg, respectively; the fusion and crystallization enthalpies of the CPCM (70 wt% PA) were 173.8 kJ/kg and 183.4 kJ/kg, respectively; the enthalpies of fusion and crystallization of the CPCM (80 wt% PA) were 182.1 kJ/kg and 193.9 kJ/kg, respectively; the enthalpies of fusion and crystallization of the CPCM (90 wt% PA) were 192.0 kJ/kg and 209.0 kJ/kg, respectively. Taking the composite material containing 20 wt% porous carbons as an example, the latent heat value was 182 J/g, which was an increase of approximately 7% compared with the theoretical latent heat value. Carbon-based materials do not contribute to latent heat; therefore, the latent heat storage capacity of PCMs was lower than that of pure PA [18]. There was a slight difference between the pure PA and CPCMs, which might have been caused by the physical interaction between PA and BPC [19, 20].
| Melting process | Crystallization process | |||||
|---|---|---|---|---|---|---|
| Sample | Melting potential (J/g) | Phase transition start temperature(°C) | Phase transition end temperature(°C) | Crystallization potential (J/g) | Start crystallization temperature (°C) | End crystallization temperature (°C) |
| PA | 212.3 | 41.19 | 50.91 | 226.4 | 42.51 | 31.46 |
| 70% PA-BPC | 173.8 | 39.25 | 50.04 | 183.4 | 41.43 | 12h |
| 80% PA-BPC | 182.1 | 40.12 | 51.36 | 193.9 | 41.36 | 30.47 |
| 90% PA-BPC | 192 | 40.81 | 49.76 | 209 | 41.25 | 29.83 |
Table 1. Thermal properties of Paraffin Wax (PA) and Biomass Porous Carbon (BPC) Composite Phase Change Materials (CPCMs)
Optical performance characterization
Organic PCMs have a high latent heat capacity; however, their photo thermal conversion capacity is weak. Researchers have generally added materials with good optical properties to PCMs to improve their optical properties. The light-to-heat conversion performance of PCMs is directly related to their ability to absorb solar energy. Some studies have also explained the thermal diffusion mechanism of the photo thermal system [21-24]. Solar light is captured by the surface of the CPCM and converted into heat energy, which indirectly increases the temperature of the entire CPCM through heat conduction in its structure to achieve heat storage [25-27].
Therefore, the selection of PCMs that can efficiently capture, store, and exchange solar energy is key to developing an efficient solar-to-light-to-heat conversion system. In this study, BPC was used as a material to enhance the light absorption properties of PA, and an ultraviolet-visible-near-infrared spectrophotometer (UV-3600 Plus) was used to accurately measure the reflectance, transmittance, and absorbance of PA and its composite materials to obtain detailed light absorption data. This rate was shown in Figure 3. The energy of sunlight consists of approximately 7% ultraviolet light (below 400 nm), 50 % visible light (400–760 nm) and 43% infrared light (above 760 nm). Visible light and near-infrared radiation account for approximately 93% of solar energy. Therefore, the transmittance, reflectance, and absorbance of CPCMs in the visible and near-infrared spectra were very important. The reflectance spectrum in Figure 3(a) showed the reflectance of PA and its composite materials in the wavelength range of 190–2000 nm. In the visible light range (400–760 nm), PA had a higher reflectivity, reaching more than 75%. However, the CPCM had a low reflectivity between 5% and 10%. The transmission spectrum in Figure 3(b) showed that the CPCM had a lower transmittance in the wavelength range of 190–2000 nm (approximately 5%). Figure 3(c) compared the absorbance between PA and its composite materials at wavelengths of 190–2000 nm, and the absorbance of the CPCM was much higher than that of paraffin over the entire spectral range, and the 70% PA-BPC CPCM had an average absorbance in the range of 190–2000 nm of approximately 1.3; furthermore, approximately 95% of the solar radiation energy was absorbed by the CPCM and stored as thermal energy. Therefore, according to the above characterization results, the prepared BPC-based CPCM had an excellent ability to capture sunlight in the visible and near-infrared ranges.
Analysis of photo thermal conversion experiment
The photo thermal conversion properties of PA and its CPCMs were studied by exposing samples with the same weight and thickness to a simulated sun with a constant light intensity of 164 W/m2 and placing thermocouples at the center of the bottom of the sample to record the temperature change at the bottom of the sample. Under solar radiation, the temperature of the sample gradually increased, as shown in Figure 4. In the PA sample absorbance heating curve, approximately 3950s elapsed before the overall temperature reached 50°C. By turning off the solar simulator switch, the temperature of the pure PA dropped rapidly, and 4850s elapsed before the system decreased to the initial temperature. Compared with that of PA, the heating rate of CPCMs was faster, and the time required for cooling was significantly reduced. As shown in Figure 4(d), the 70% PA-BPC CPCM increased from room temperature to 50°C in 1500’s under simulated sunlight, in contrast to the 3950’s required for pure PA. The light-to-heat conversion performance significantly improved. The temperature decreased from 50°C to 30°C in the absence of light; pure PA required 4850s for this decrease, but 70% PA-BPC only required 3600s, which was 1250’s shorter and reflected the thermal conductivity of the material was greatly improved.
In addition, based on the results of the aforementioned temperature change as a function of time experiment, the light-to-heat conversion efficiency (η) was calculated according to the following formula [7]:
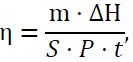
where m is the mass of the PCM, g; S is the area of the upper surface of the CPCM, m2; ΔH is the phase transition enthalpy (latent heat value) of the CPCM, J/g; P is the intensity of simulated sunlight, W/m2; and t is the phase transition time of the CPCM, s. The photo thermal conversion efficiencies (η) of 70% PA-BPC, 80% PA-BPC, and 90% PA-BPC were 89.6%, 85.6%, and 72.5%, respectively, showing high photo thermal conversion ability.
To study the heat production and heat transfer of CPCMs absorbing solar photons under simulated sunlight, the photo thermal conversion process of PCMs under continuous sunlight was observed using an infrared thermal imager, and the change in the internal temperature of CPCMs with illumination time was observed.
Figure 5 shows a schematic of the photo thermal conversion experiment. The prepared CPCM was pressed into a cylindrical mold. A solar simulator xenon lamp was placed directly above the sample. When the solar simulator was turned on, the mold was irradiated using a xenon lamp. The front surface of the sample was subjected to photo thermal conversion. An infrared thermal imager (FLIR T640, Teledyne FLIR, Wilsonville, OR, USA) was placed in front of the sample to collect the real-time temperature distribution diagram of each sample during the illumination process.
Figure 6 is the temperature distribution diagram of each sample at 0 s, 20 min, 40 min, and 60 min (from Figure 6). From left to right are pure PA, 90% PA-BPC, 80% PA-BPC, and 70% PA-BPC). As shown in Figure 6, BPC effectively converted sunlight into heat energy. After 20 min of illumination, the surface of the CPCM absorbed a large amount of heat energy, and the PCM began to melt when the temperature reached 50–60 °C. However, there was almost no change on the upper surface of pure PA, which indicated that pure PA exhibited almost no light absorption in the visible light range.
Comparing the temperature distribution of different CPCMs indicated that the overall temperature of the material increased with increasing amounts of BPC. This result occurred because the thermal conductivity increased with an increase in the BPC content, resulting in the enhancement of the light absorption capacity and acceleration of the heat transfer rate, and the overall temperature was improved.
The temperature distribution of the CPCM is shown in Figure 6. From the top to the bottom of the sample material, the temperature decreased in a gradient distribution. The top was closest to the simulated solar light source, absorbing a large amount of solar light and generating a large amount of heat to increase the top temperature. Because heat could not diffuse from the top in a timely manner, an obvious temperature difference appeared. The overall temperature of the sample increased over time.
Potential applications of solar CPCMs
China has established solar energy as key to the development and utilization of renewable energy within an energy development planning framework. This type of CPCM with good optical properties had a high latent heat capacity and suitable phase change temperature, making this material a good low-temperature energy storage material. Applying this material to solar power applications, such as solar collectors, to solve the problem of intermittent solar radiation provides a huge opportunity to increase energy supply and demand. CPCMs can store excess solar energy during non-sunlight hours [13]. Similarly, these materials can be used in industrial waste heat recovery systems to store a large amount of waste heat that is used for indoor or space heating in a later period, and for medical equipment, such as solar thermal compression bags, it can be compared to traditional thermal compression bags. These materials easily recycle and recharge under sunlight. In addition, compared with recently reported solar-CPCMs, as shown in Table 2, the CPCMs in this study were more feasible and competitive in solar thermal conversion. However, in order to make large-scale applications of solar-CPCMs, more research is needed [28].
| Sample | Load (%) | Latent heat value (J/g) | Light-to-heat conversion rate (%) | Source |
|---|---|---|---|---|
| Stearyl alcohol/graphene-based aerogel | 13.3 | 196.2 | 84 | [1] |
| Paraffin/biomass Aerogel | _ | 115.2 | 71.4 | [29] |
| Phase change microcapsule/GO | _ | 234.7 | 76.03 | [30] |
| Phase change polymer matrix/functionalized porous carbon | _ | 180.3 | 72.1 | [14] |
| Paraffin/BPC | 30 | 173.8 | 89.6 | This work |
Table 2. Comparison of optical properties between reported Composite Phase Change Materials (CPCMs) and the Biomass Porous Carbon (BPC) composites prepared in this paper.
In this study, biomass materials were prepared into biomass carbon-based materials by high-temperature carbonization and added to organic PCMs to form an effective solar CPCM. Compared with that of pure PA, the thermal conductivity of the CPCM (70% PA-BPC) increased by 3.18 times, and the average absorbance in the range of 190–2000 nm was close to 1.3. Approximately 95% of solar radiation passed through the composite phase. A variable material absorbed and stored this radiation as thermal energy. A solar simulator was used to illuminate the top surface of the sample vertically, and the surface of the CPCM captured sunlight, which caused the temperature to rise. The heat at the top could not diffuse downward over time, causing a significant temperature difference between the upper and lower sides. As time passed, the overall temperature of the sample increased. As the amount of BPC added was increased, the overall rate of the temperature rises of the material increased. This result occurred because, as the content of BPC increased, the light absorption performance and thermal conductivity of the composite PCMs increased the heat transfer rate and improved the overall properties of the sample. In the photo thermal conversion experiment, compared with those of pure PA, the 70% PA-BPC CPCM had a faster heating rate, and the time required for cooling was greatly reduced. The maximum photo thermal conversion rate of the 70% PA-BPC CPCM was 89.6%. The synergy of the thermal conductivity, solar thermal conversion capability, and latent heat of the CPCM can serve as the basis for the application of solar photo thermal conversion and storage technologies.
The authors would like to acknowledge the Zhongyuan science and technology innovation talents (234200510011) and Key Technologies R&D Program of He’nan Province (232102321089) and Collaborative Innovation Program of Zhengzhou (Major and Key Program of ZZULI) (2021ZDPY0107).
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]