ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Varsha Gautam1, Loni Lokwani2, Sharda Sharma2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
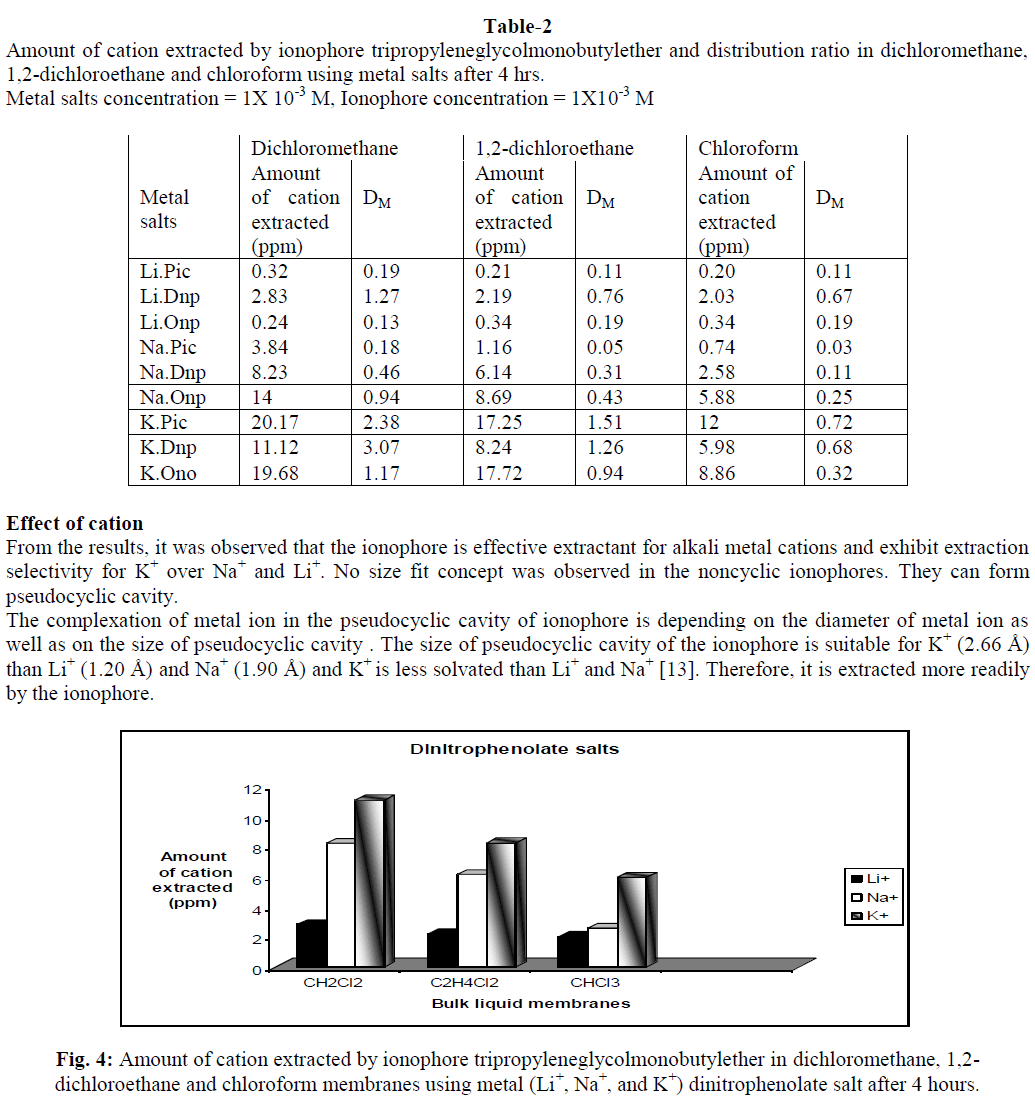
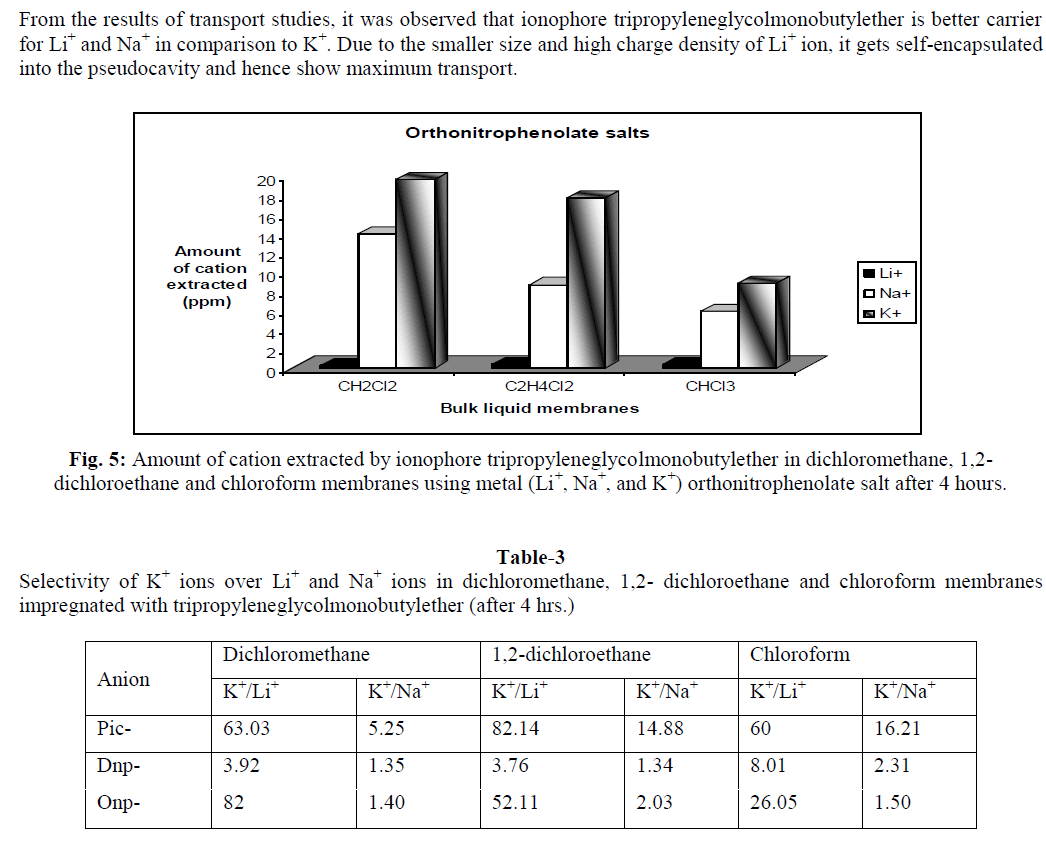
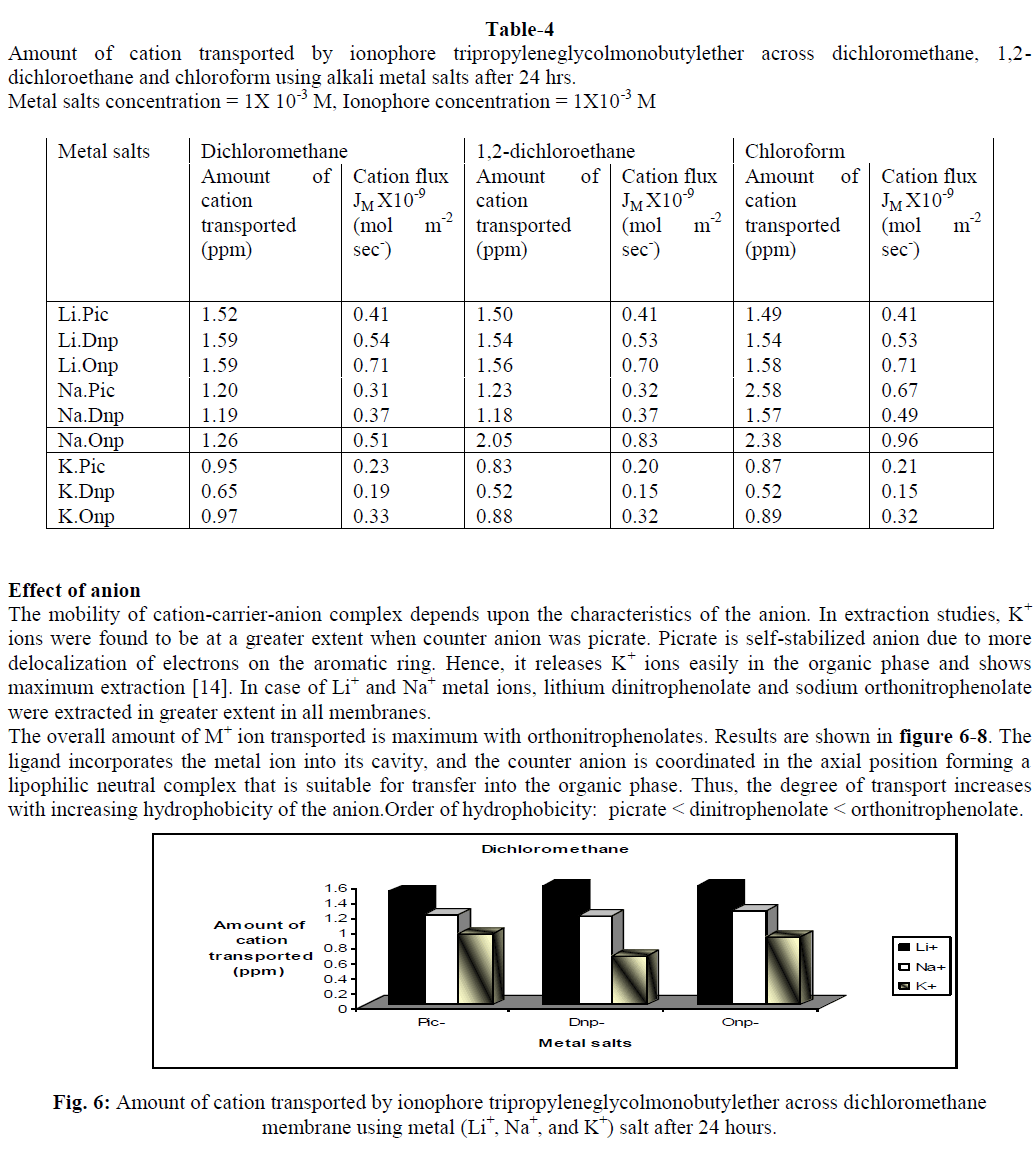
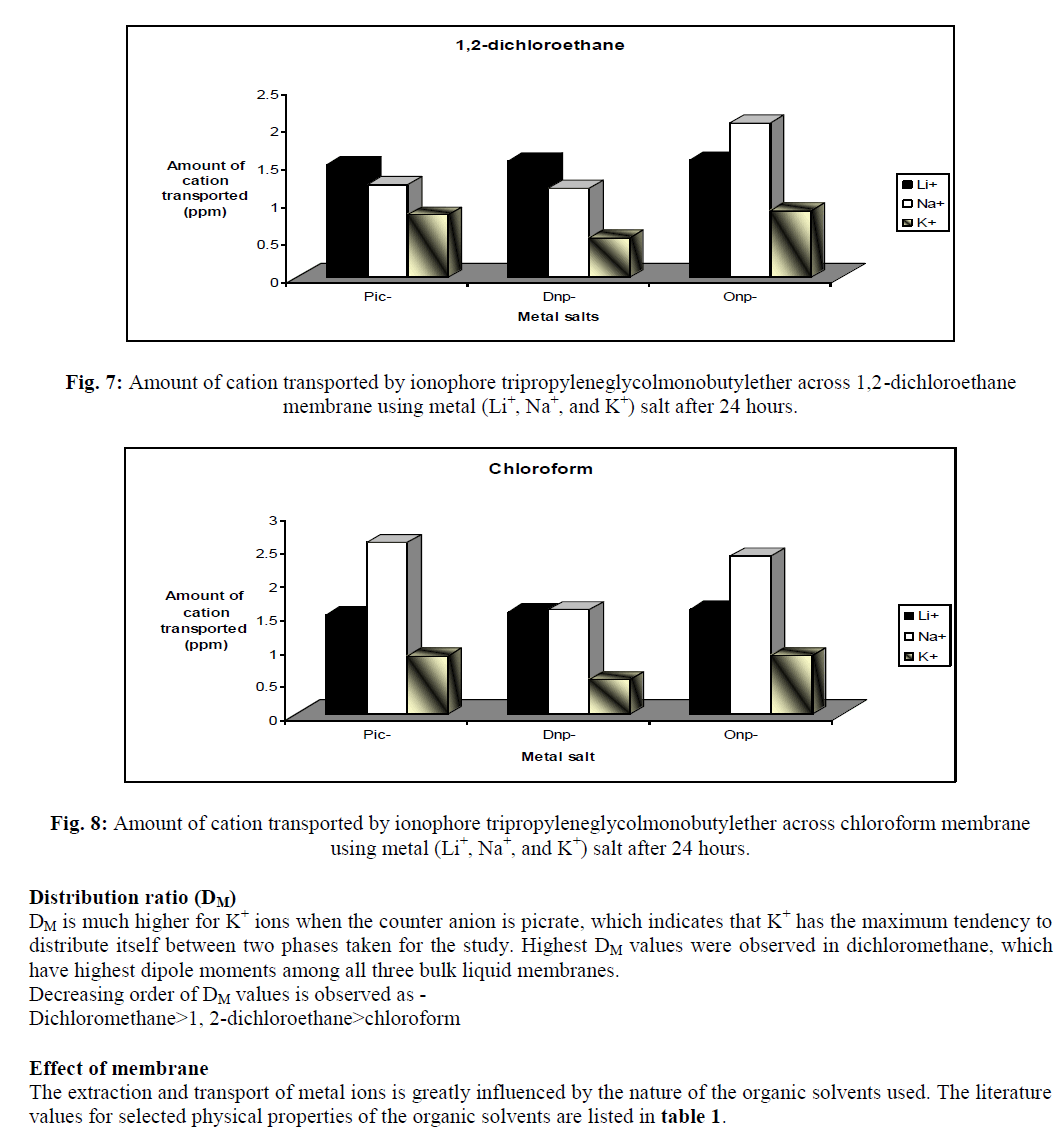
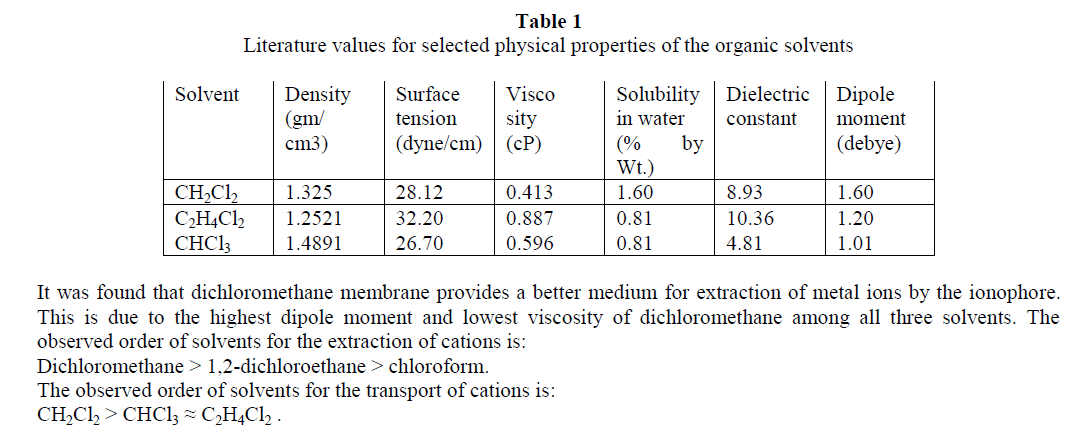
In supramolecular chemistry, liquid membranes are frequently used to evaluate the complexation and transport properties of receptors. Tripropyleneglycolmonobutylether (TPGMBE) was used to study extractability and selectivity for alkali metal ions (Li+, Na+ & K+) using picrate (Pic-),dinitrophenolate (Dnp-) and orthonitrophenolate (Onp-) salts through various organic membranes viz. dichloromethane, 1,2-dichloroethane and chloroform. Higher extraction values were observed for K+ ions from picrate salts while Li+ ion shows maximum transport ability using onp- as anion. Dichloromethane was found to be the best membrane for both extraction and transport processes. Liquid membrane technology provide applications in small and large separations, drug delivery and electrode sensing techniques etc.
Keywords |
| Complexation, Alkali metal ions, Transport, Extraction and TPGMBE. |
INTRODUCTION |
| Separation phenomenon through molecular recognition of the host compound has been widely used by incorporation of ionophore into solvent extraction of liquid membranes [1]. The molecular recognition of Na+ and K+ ions by membraneintegrated ligands is one of the fundamental processes of living cells [2]. |
| Complex formation is a very important process in which bonding interaction occurs. Molecular recognition is based on selective and specific complexation routes. This selective behavior is very important in the case of drug consumption in the body, where the drug selectivity and its selective interactions are vital [3]. Solvent extraction of metal has been extensively used in hydrometallurgical process. Metal salts usually are not soluble in organic solvents. Hence, this process required the introduction of an extractant that will combines with the metal ion to form an organic soluble species [4]. Membrane separation in bioreactors is one of the most attractive operations applied in biochemical processes [5]. Selective purification and concentration of metals in solution has primarily been achieved using liquidliquid extraction techniques [6]. |
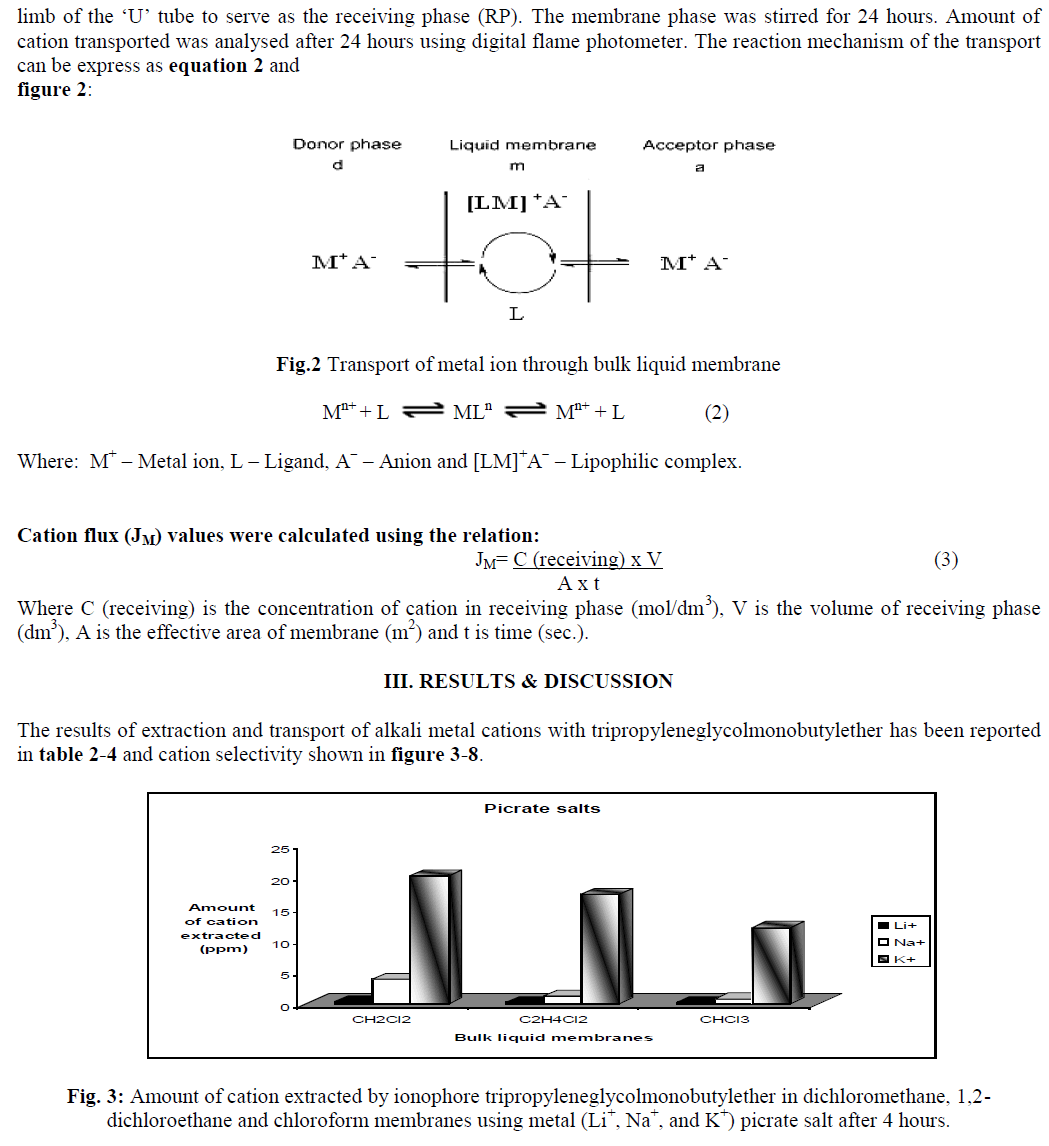
| Carrier assisted transport through liquid membrane is one of the important applications of supramolecular chemistry. Non-cyclic ionophores (Podands) show binding and selectivity towards alkali and alkaline earth metal ions. This work investigates the use of synthetic carrier for the separation of metal ions [7]. Trans-membrane transport system using synthetically created membrane incorporating ionophores are biologically inspired in the sense that they mimic wellknown biological process such as facilitated ion transport using ion channels like Na+ - K+ ion pumps [8]. Extraction studies were conducted to ascertain the occurrence or complexation between metal salt and the ionophore in solution state [9]. |
| In this paper, we are reporting here the liquid-liquid extraction and carrier facilitated transport of Li+ Na+ and K+ picrates, dinitrophenolates and orthonitrophenolates by tripropyleneglycolmonobutylether through bulk liquid membrane system using dichloromethane, 1,2-dichloroethane and chloroform membranes. |
MATERIALS AND METHODS |
| Materials |
| The metal salts (MX) in the form of picrate (Pic-), dinitrophenolate (Dnp-) and orthonitrophenolate (Onp-) [MX: M+ = Li+, Na+ and K+; X Ãâ¹Ãâ° = Pic-, Dnp- and Onp-] were prepared by the reported method [10]. Dichloromethane, 1,2- dichloroethane and chloroform were obtained from Merck and Qualigens and used without further purification. Analytical grade chemicals were used. Ionophore tripropyleneglycolmonobutylether [figure 1] was purchased from Aldrich. |
| Instruments |
| In all experiments, the instruments employed were digital flame photometer (Systronics – 128) for Li+, Na+ and K+ estimation, magnetic stirrer (Model Remi – 2 MLH) and analytical balance (A X 200) of SHIMADZU Corporation, Japan and melting point apparatus (MAC). |
| Methods |
| Liquid-liquid extraction studies |
| To investigate the carrier-facilitated extraction, 10 ml of 1.0 x 10-3 M aqueous salt solution was stirred with 10 ml of 1.0 x 10-3 M ionophore solution in bulk liquid membrane viz. CH2Cl2, C2H4Cl2 and CHCl3, in a covered small beaker using a magnetic stirrer (200 rpm) [11]. The amount of cation in aqueous phase was initially determined before extraction was conducted using flame photometer. After 4 hours of stirring, the mixture was allowed to stand for 5 min. for separation of two phases. The depleted aqueous phase was removed and analyzed for residual concentration of metal ions using digital flame photometer. |
| The amount of cation extracted by ionophore was found by determining its difference in aqueous phase before and after extraction. A blank experiment was also performed simultaneously with the same to determine the leakage of metal ion from aqueous to organic phase in the absence of carrier. All measurements were performed in duplicate to check the reproducibility. Values of distribution ratio (DM) were calculated as equation 1: |
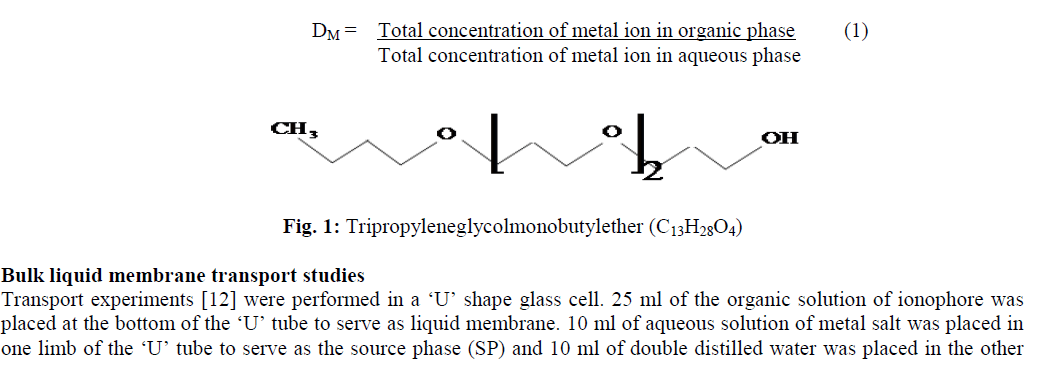 |
 |
 |
 |
 |
 |
 |
CONCLUSION |
| The present study demonstrates that the ionophore tripropyleneglycolmonobutylether is an excellent carrier for extraction and transport of alkali metal ions. Extraction of metal ion depends upon the nature of anion, metal-ligand interactions and conformation of ionophore used. It was observed from the studies that this ionophore is good extractant for K+ ions from picrate salt while during transport studies it is better carrier for Li+ ions from orthonitrophenolate salts. The selectivity observed for K+ ions among these biologically important ions (Li+, Na+ and K+) may also be of much importance as models for cation transport across biomembrane. It provides good applications for the development of Li+ ion specific receptors, which can help in monitoring of lithium concentration in human body and in ion selective electrode. |
ACKNOWLEDGEMENT |
| We are thankful to the University Grant Commission, New Delhi for the financial support in the form of minor research project. We are also thankful to Department of Science & Technology, New Delhi for providing the instrumental facilities to the Department of Chemistry, Government College, Kota (India) under FIST programme. |
References |
|