ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| H.N.Dash, D. Rout and B.B.Kar School of Applied Sciences, KIIT University, Bhubaneswar |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Vanadium has got various industrial uses. Being a refractory material, its disposal is leading to environmental contamination all the time. Recovery of vanadium pentoxide from nearby detergent factory was performed using a two-step process involving high temperature treatment followed by adsorption. Finally, NaOH was used in various concentrations, solid to liquid ratios, stirring times and temperatures. A high solid/liquid ratio in the leaching stage was used to obtain high concentration of vanadium pentoxide and low acid consumption that allowed direct precipitation without the use of extraction by rather expensive organic solvents. Sodium hydroxide solution of one mole/liter concentration was used in the precipitation stage. The adsorption studies were carried out to optimize the maximum recovery of vanadium.
Keywords |
| catalyst, leaching, precipitation, vanadium |
INTRODUCTION |
| Vanadium is having a wide application in industry. Today over 90% of the world production of vanadium is consumed in the production of stainless steel as an alloying agent to produce ferro-vanadium. Vanadium is employedfor the manufacture of a variety of vanadium compounds, many of which in turn are employed to prepare catalysts such as hydrocarbon oxidation catalysts and catalysts for the manufacture of detergent [1]. Some authors have stated that vanadium could be recovered from the detergent industry waste solutions derived from the leaching of spent catalysts. Another group of researchers [2] described a process for the recovery of vanadium from sulphate leaching solution using organic phase containing di(2-ethylhexyl) phosphoric acidcombined with tributyl phosphate. Scientists have [3] compared the di(2-ethylhexyl) phosphoric acid and amine extractants and investigated the relative extracting performance of quaternary amine in the case of a range of vanadium (V) anionic species between pH 6 and 13. Alkaline leaching is more selective for iron but dissolves some silica and is more costly in terms of reagent [4]. Researchers have [5] describes a process in which vanadium and molybdenum were recovered as sodium vanadate [(NH4)3VO4] and H2MoO4, with yields of 96% and 92%, respectively. The purpose of the present work is to study the possibilities for the recovery of vanadium pentoxide by dissolving the spent catalyst with suitable acid media and oxidize the vanadium to higher oxidation state, then precipitate it directly by converting the vanadium into simple vanadate compounds which is insoluble in the pH adjusted solution without the use of relatively expensive organic extraction solvents. |
II.EXPERIMENTAL PROCEDURE |
| Materials Required: The spent vanadium catalyst is obtained from the prime Detergent factory, Rasulghar, Bhubaneswar. Here, sulphur trioxide is obtained through the preparation of sulphuric acid.The additional reagents used are of anal an R grade. |
 |
| In the present process, the vanadium oxide could be obtained using soda ash roasting method.Initially, the spent catalyst is ground to 106 μm mesh size and is used as the raw material. About 50 mgs of the spent catalyst powder is to be kept inside the porcelain crucible to which about 50 ml of 80%. NaOH is addedto prepare an uniform mixture. This crucibleis kept inside the furnace at 200 0C for 5hrs retention period. The roasted sample was then transformed to a beaker containing water and leached for 60 mins with water to solid ratio 10:1. |
III.THEORY |
 |
IV.RESULT AND DISCUSSION |
| The study revealed the effect of various process parameters on the extraction of vanadium an contaminated catalytic source. In this aspect the role of temperature, NaOH concentration solid to liquid ratio, loading time are being studied extensively. |
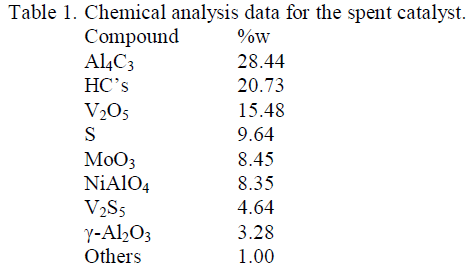
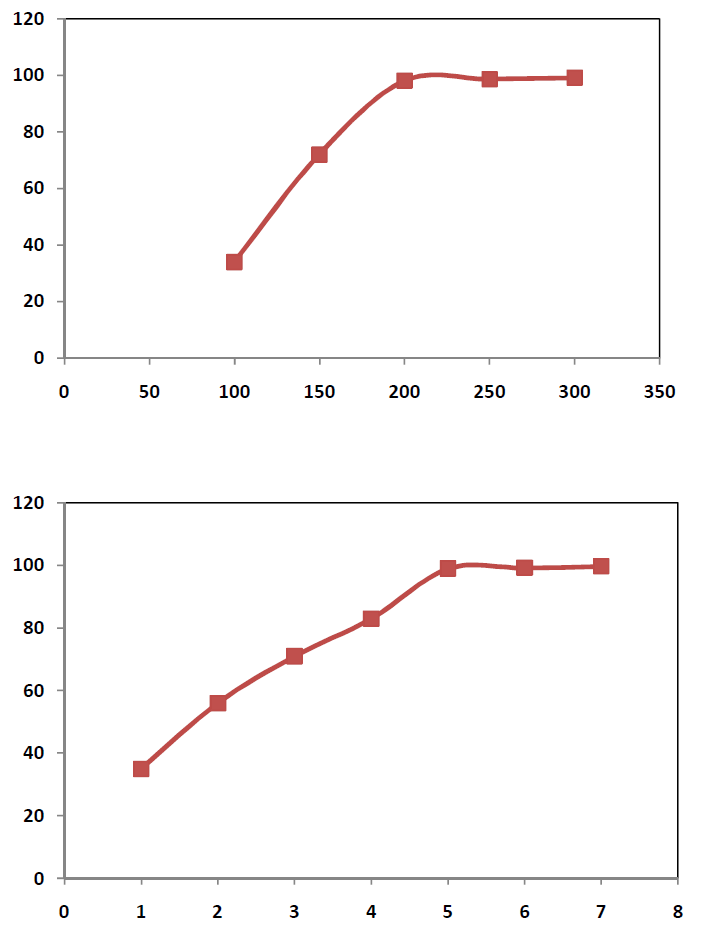
| Role of temperature In order to study the role of temperature, 50 gms of sample was taken in each run along with 80% NaOH in the ratio 1:1. The heating time was kept 5hrs and the roasting temp was varied between 100 0C to 300 0C with an internal of 50 0C. The roasted product was then transferred to a 1000 ml beaker containing 500 ml water. The percentage vanadium extracted is represented in Figure-I. The study revealed that 200 0C temperatures is found to be sufficient enough to extract about 98% of vanadium from vanadium bearing spent catalyst. Role of Retention time In order to study the role of time on vanadium extraction, attempt has been made to conduct experiments at 200 0C temperature in which the solid to liquid ratio during roasting is kept at 1:1. During the study, 80% NaOH solution is used as the activating medium. The time duration various from 1 hr to 7hrs. The data obtained are incorporated in figure- II.The data revealed that the sample bearing NaOH and spent catalyst, when roasted at 200 0C for 5hrs time could produce 99 % Vanadium that could be extracted successively. Effect of NaOH Concentration The effect of Concn of NaOH on studied using 1:1 solid as liquid ratio, 5hrs time and 200 0C roasting temperature where NaOH concn was varied drastically. The result obtained are represented as Figure-III.The result shows that maximum up to 20% NaOH is ideal enough to extract about 99% of Vanadium from the spent catalyst. The result shows that maximum up to 80% NaOH is ideal enough to extract about 99% of vanadium from the spent catalyst. |
V.CONCLUSION |
| The above study revealed that it is possible to extract vanadium from Vanadium condition for extraction of maximum percentage of vanadium is found to be 80% of NaOH when mixed with the spent Vanadium catalyst in the ratio 1:1 and roasted out 200 0C for 5hrs time, it is possible to extract about 99% Vanadium from the catalyst.The vanadium was obtained in the form of brown red precipitate, which is being filtered and analyzed. This Vanadium through activated carbon could produce Vanadium carbide. |
 |
References |
|