ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Dr. Inaam M. Abdulmajeed1, Dr. Fadel A. Chyad2, Dr. Muna M. Abbas3, Hadi Attya Kareem4
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The design of advanced ceramics depends on the availability of powders with outstanding properties in terms of composition, purity, size, and size distribution. Sol-gel processes allow synthesis powders to have a more elaborate structure. This paper discusses the synthesis of ultra fine particles of ZnO and MgO. At first, fine particles were synthesized by the reaction of Zn (CHCOO3)2 and Mg (CHCOO3)2 with Sodium Hydroxide. The effects of different parameters on particle size and morphology of final ZnO and MgO powders ultra fine powder were optimized by sol gel method. Under optimum conditions, uniformed and homogeneous nanostructured zinc oxide and magnesium oxide powders were obtained with particle sizes of (70–190) and (55-150) nm, respectively. Sol gel monocomponent powders have been obtained in MgO, ZnO. The structures and morphologies of MgO and ZnO particles have been characterized by X-ray diffraction (XRD), scanning probe microscope (SPM) method. Ultra fine sized powders with a tendency towards aggregation have been obtained. The tendency towards crystallization underlines the high reactivity of these powders. Such powders may be used as pigments, catalysts, supports in chromatography or as raw materials for advanced polycrystalline ceramics.
Keywords |
| Nanoparticles, Sol gel, MgO, ZnO, XRD. |
INTRODUCTION |
| During the last few years, synthesis of nanostructured oxide materials have been attracted considerable attention, Oxide semiconductor nanostructures have been widely investigated in recent years because of their excellent properties [1–3]. The metal oxides are extremely important technological materials for use in electronic, chemical or mechanical industries, as well as in relevant technologies, including superconductors, catalysts, magnetic materials, structural, and engineering materials [4]. Due to their excellent nonlinear coefficient and low leakage current, they have been used in electrical and electronic systems such as surge protection devices for many years [5]. |
| In recent years, researchers have focused more on the synthesis of ultra fine particles of ZnO and MgO due to their application in advanced technologies. The experimental conditions used in the preparation of these materials play an important role in the particle size of the product [6, 7]. Magnesium Oxide (MgO) is one of the most useful and promising material in the field of nanosized particles; the large surface area to volume ratio and the presence of reactive sites on the surface make MgO nanoparticles suitable for uses in a number of organic heterogeneous catalyst, Other possible fields of application are gas and humidity sensors and cryosurgery, due to low cost, electro-stability, nontoxic, and biodegradable properties of MgO nanoparticles.[8]. |
| Sol-gel processes allow synthesis of powders with a more elaborate structure from point view of composition, purity, size and size distribution. In the powders case, sol-gel refers to processing in a liquid medium to obtain a solid matter which does not settle under gravity that is to say which does not precipitate. The advantages of sol-gel powders comparative with conventional powders are, the small average particle size and narrow size distribution, in which case they can be called monodispersed; homogeneity at molecular level, purity and increased reactivity (which determine low processing temperature), and possibility to obtain nano-sized powders [9]. |
| The crystal structure of magnesium oxide is cubic, which makes it a periclase structure. Its atomic arrangement is of the NaCl type, and this arrangement causes the common morphologies of MgO to be cubic [10]. General, Zinc oxide crystallizes in three forms: hexagonal wurtzite, cubic zinc blende, and the rarely observed cubic rocksalt. Hexagonal wurtzite is the main stable crystal structure of ZnO at room temperature., and thus most common [11]. |
| This project work was focused on the following steps: |
| 1. Preparation of the MgO, ZnO nanoparticles by sol-gel method. Zinc acetate dehydrates and triethanolamine are the precursor’s materials here. |
| 2. Characterize the crystals structure and pure phase by using XRD diffraction patterns. |
| 3. Characterize the surface morphology both of the ZnO and MgO nanoparticles by scanning probe microscopy |
EXPERIMENTAL WORK |
| Zinc oxide and Magnesium oxide nanoparticles were synthesized using zinc acetate and magnesiumacetate tetrahydrate respectively as a source material with sodium hydroxide. All chemical used for this syntheses were purchased from Analar. 1 M of zinc acetate and magnesiumacetate tetrahydrate was prepared, where each one was dissolved in 100 ml of distilled water; then one mole of citric acid was added (stoichiometric ratios was selected for the reaction). In order to forming a homogenous sol, the solution was heated at 70 o C by a magnetic heater stirrer. After that, the solution was cooled and its pH was adjusted by added drop sodium hydroxide solution wise to the prepared. After adjusting the pH, the precipitate were filtered and washed three times to remove ionic impurities, then was heated again up to 100o C. ZnO powder sintered at 600 °C for 2 hr, but MgO powder sintered at 1100°C for 2 hr. Particle size measured using SPM made in USA. XRD measurements were used to calculate the structure of MgO and ZnO ultrafine powders. |
RESULT AND DISCUSSION |
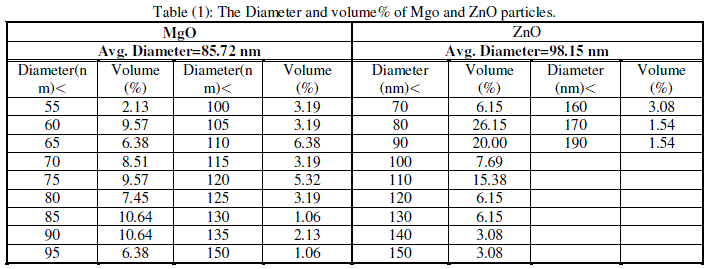
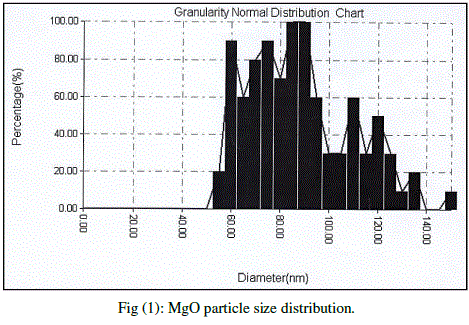
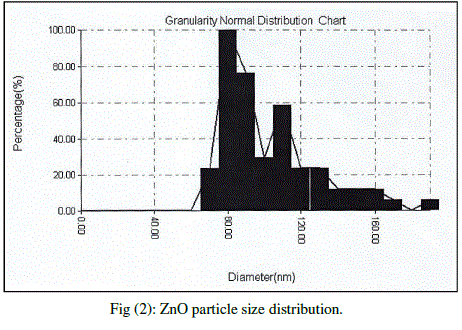
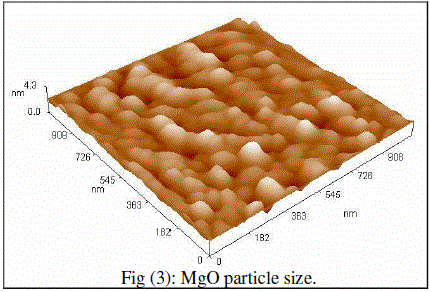
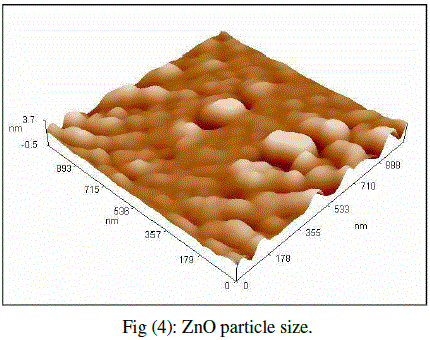
| Cubic shaped Magnesium oxide nano and ultra fine particles were successfully synthesized by sol-gel method using magnesium nitrate and sodium hydroxide. The MgO prepared powder is ultra fine although some agglomeration is revealed in Fig.(1), but most of the agglomerated particles still have diameters less than 150 nm sized and although some agglomeration is revealed in Fig.(3), also shows the particle size is in the 55-190 nm range. The average particle size of synthesized powders was 85.72 nm. Hexagonal ZnO particles were prepared by sol gel method, the smaller particles are about 70 nm in diameter, and larger particles exceed 190 nm Fig.(2). On the other hand, the average particle size of synthesized powders was 98.15 nm and the powder shapes were almost uniform as shown in Fig (4). |
 |
 |
 |
 |
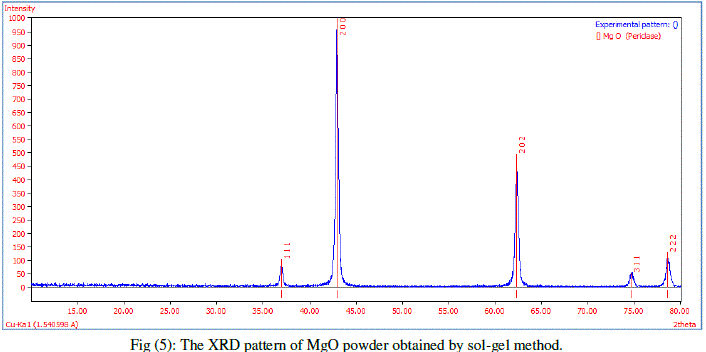
| X-ray Powder Diffraction (XRD) studies were carried out to confirm the crystallinity using X-ray diffractometer with cukα radiation (λ = 1.5418 Å) in the range of 10–80° to determine their crystal structure and phase Figures (5), illustrates a typical XRD spectrum of MgO nanoparticles prepared by sol jel method. Five diffraction peaks were seen at 36.92, 42.9, 62.28, 74.65 and 78.6, which can be assigned to diffraction from (111), (200), (202), (311) and (222) planes respectively. Structure magnesium oxide with the lattice parameter a = 4.241 Å, the diffraction peaks of MgO can be matched with standard Joint Committee on Powder Diffraction Standards-JCPDS data [JCPDS file: 45-0946]. |
 |
| X-ray diffraction patterns indicate that the obtain ultra fine particles are in good crystallinity, The XRD spectra indicate that the ZnO crystal has a hexagonal wurtzite structure, the major diffraction peaks were seen at 31.76, 34.6, 36.25, 47.53, 56.60, 62.86, 66.37, 67.96, 69.09 and 76.95, which can be assigned to diffraction from (100), (002), (101), (102), (110), (103), (200), (112), (201), and (202) planes respectively as shown in Fig. (6). |
 |
CONCLUSION |
| In this study, the following results were obtained: |
| 1. Ultra fine and nano powders of zinc oxide and magnesium oxide with 98.15 nm and 85.72mm average grain size respectively were synthesized by a sol-gel method. |
| 2. Cubic MgO and hexagonal wurtzite ZnO crystal structures were successfully synthesized by sol-gel method. |
References |
|