e-ISSN: 2319-9849
e-ISSN: 2319-9849
Department of Chemistry, Govt Degree College, Kodur (RS) YSR Kadapa Distict, Andhra Pradesh, India
Received: 07/03/2013; Revised: 13/03/2013; Accepted: 20/03/2013
Visit for more related articles at Research & Reviews: Journal of Chemistry
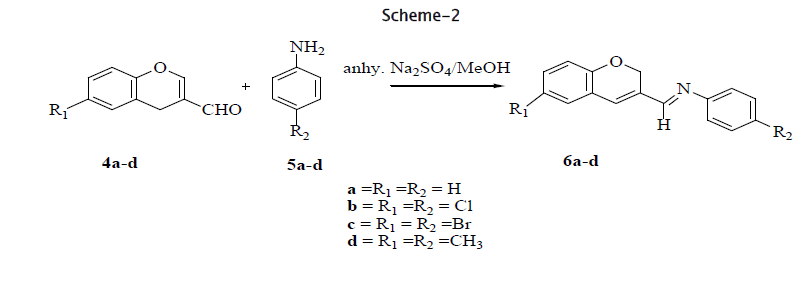
The Claisen-Schmidt condensation of 3,5-dibenzylidene-4-one(1a-f) react with propargylbromide(2) in presence of K2CO3 at room temperature to gave 3,5-dibenzylidene-1-prop-2-ynyl-piperidin-4-ones(3a-f), and 2H-3-chromenecarbaldehydes (4a-d) react with aniline (5a) and anhydrous Na2SO4 to give 2H -chromene-3-(4'-pheny1) imine (6a-d) in good yields.
Claisen-Schmidt condensation, 3,5-dibenzylidene-4-one,propargylbromide, 2H-3-chromenecarbalehydes, aniline.
Heterocyclic ring systems having piperidin-4-one nucleus have aroused great interest in the past and present years due to their wide range of biological activity such as anti viral, and anti microbial activity and their derivative piperidines are also biologically important and act as neurokinin receptor antagonists [1,2,3,4]. The bis (substituted benzyliden) cycloalkanones are very important precursors to potentially bioactive pyrimidine derivatives intermediates of agrochemicals, pharmaceuticals, and perfumes new organic materials for nonlinear optical applications, cytotoxic analogues and the units of liquid-crystalline polymers [5,6,7]. In addition these compounds undergo double 1,3-dipolar cycloaddition reaction with azomethine to give bis-spiropyrrolidines, which are often the central ring system of numerous natural products.
General Methods
Melting points were determined on a Polmon instrument (model no. MP-96).IR spectra were recorded on Perkin-Elmer 337 spectrometer, and 1H NMR (400 MHz) and 13C NMR (100.6 MHz) were recorded on a Varian Gemini 200 spectrometer using TMS as internal standard (chemical shifts values were described in ppm δ). Mass spectra were recorded on a VG micromass LCMS 2010 instrument.
I.General procedure for the synthesis of 3, 5 - dibenzylidene-1-prop-2-ynyl-piperidin-4-ones (3a-d) [8,9,10].
3,5-Dibenzylidene-piperidin-4-one (1) (6.87 gr,25 mmol) dissolved in acetone (25mL), propargylbromide (2) (4.13 gr, 35 mmol), K2CO3 (6.9 gr, 50 mmol) was added and stirred at room temperature for 8 hours. Acetone was decanted, concentrated and then ice cold water was added. The solid obtained was separated on column chromatography with petroleum ether: ethyl acetate (9:2) to give 3,5-dibenzylidene-1-prop-2-ynyl-piperidin-4-one (3a).
i.3,5-Dibenzylidene- 1 -prop-2-ynyl-piperidin-4-one(3a).
Yield: 75%, mp: 157 °C
IR (KBr): 2900 cm-1, 2100 cm-1, 1650 cm-1.
1HNMR (400 MHz) δ 2.32(t, lH, J=2.4Hz, - C=CH) 3.51 (d, 2H J=2.4 Hz, -CH2-C=), 3.89 (s, 4H, piperidinone ring-H), 7.38 (m, 10H, Ar-H), 7.75 (s 2H arylidene-H).
MS: m/z 314 (98) [M+H]+.
ii.3,5-Bis-(4-chloro-benzylidene)-1-prop-2-ynyl-piperidin-4-one(3b).
Yield: 77%, mp, 136 °C
1HNMR (400 MHz) δ 2.51 (t, 1H, J =2.6Hz, -C=CH), 3.53 (d 2H, J =2.6Hz, -CH-C=), 3.84 (s, 4H, piperidinone ring-H), 7.54 (m, 8H, Ar-H), 7.60 (s, 2H, arylidene-H).
MS: m/z 382 (78) [M+H]+
iii. 3,5-Bis-(4-methoxy-benzylidene)-1-prop-2-ynyl-piperidin-4-one (3c).
Yield: 80%, mp: 130 °C
1HNMR (400 MHz) δ 2.67 (t, lH, J = 2.0 Hz, -C=CH), 3.85 (d, 8H, J = 2.4 Hz, -OCH3, & - CH2-C=), 3.89 (s, 4H, piperidinone ring-H), 6.99 (d, 4H, Ar-H), 7.40 (d, 4H, Ar-H) 7.66
(s, 2H, arylidene-H).
MS: m/z 374 (89) [M+H]+.
iv.3,5-Bis-(4-Bromo-benzylidene)-1-prop-2-ynyl-piperidin-4-one(3d).
Yield: 77%, mp: 136 °C
1HNMR (400 MHz) δ 2.53 (t, 1H, J =2.6Hz, -C=CH), 3.53 (d 2H, J =2.6Hz, -CH-C=), 3.84 (s, 4H, piperidinone ring-H), 7.38 (m, 8H, Ar-H), 7.19 (s, 2H, arylidene-H).
MS: m/z 487 (98) [M+H]+
v. 3,5-Bis-(4-methyl-benzylidene)-1-prop-2-ynyl-piperidin-4one (3e).
Yield: 80%, mp: 130 °C
1HNMR (400 MHz) δ 2.12 (t, lH, J = 2.0 Hz, -C=CH), 3.25 (d, 8H, J = 2.4 Hz, -CH3,), 3.89 (s, 4H, piperidinone ring-H), 6.95 (d, 4H, Ar-H), 7.01 (d, 4H, Ar-H) 7.66 (s, 2H, arylidene-H).
MS: m/z 357 (89) [M+H]+.
vi. 3,5-Bis-(4-ethoxy-benzylidene)-1-prop-2-ynyl-piperidin-4-one (3f).
Yield: 80%, mp: 130 °C
1HNMR (400 MHz) δ 2.67 (t, lH, J = 2.0 Hz, -C=CH), 1.33 (d, 8H, J = 2.4 Hz, -CH3, & - 3.98, CH2), 3.89 (s, 4H, piperidinone ring-H), 6.72 (d, 4H, Ar-H), 7.190 (d, 4H, Ar-H) 7.66 (s, 2H, arylidene-H).
MS: m/z 417 (100) [M+H]+.
II. General procedure for the synthesis of 2H-3-chromeneimines (6a-d) [11,12,13,14,15,16,17,18,19,20]
A mixture of 2H-3-chromenecarbalehyde (4a) (1.6g, l0mmol), aniline (5a) (1.23g, l0mmol) and anhydrous Na2SO4 (3.0g) was refluxed in dry methanol (50mL) on water bath for 6 h. After completion of the reaction the solution was decanted and methanol was evaporated under reduced pressure. The crude brown colored reaction mass was subjected to column chromatography over neutral alumina and elution with pet.ether:ethyl acetate (9:1) gave 2H-chromene-3-(4'-pheny1) imine (6a) as a light brown solid (2.1g. 80% yield), mp 117 °C
Employing the similar procedure as mentioned 6a, compounds 6b-d were obtained from 4b-d.
IR (KBr): 1630cm-1(C=N) and 1578cm-1(C=C). UV (MeOH): 334 nm (log ε 4.2), 281 nm (log ε 4.5) and 248 nm (log ε 4.3). 1H NMR (400 MHz): δ 8.13(s, CH=N), 7.01-7.18(m, H-5, 7; H-2', 6ʹ), 6.78-6.90(m, H-4, 6, 8; H-3', 5ʹ), 5.22(s, 2-OCH2). 13C NMR (100.6 MHz): δ 158.4(C-4'), 155.7(CH=N), 155.2(C-8a), 144.4(C-1), 132.0(C-7), 131.7(C-3), 130.8(C-5), 127.9(C-4), 122.2(C- 2',6'), 121.8(C-4a), 121.5(C-6),116.0(C-8), 114.4(C-3',5'), 65.0(C-2). MS: m/z 235(M+) (100).
viii) 6-Chloro-2H-chromene-3-(4'-chlorophenyl)imine (6b)
Light yellow needles; yield 80-85%, mp 98 °C.
IR (KBr): 1629 cm-1(C=N) and 1574 cm-1(C=C).
UV (MeOH): 343 nm (log ε 4.4), 276 nm (log ε 4.3) and 241 nm (log ε 4.5).
1H NMR (400 MHz): δ 8.15(s, CHN), 7.15-7.31(m, 14-5, 7; H-2', 6ʹ), 6.89-7.00(m, H-4, 8), 6.85(d, J=9.0Hz, H-3', 5ʹ), 4.57(s, 2-OCH2).
13C NMR (100.6 MHz): δ 158.4(C-41), 155.0(CH=N), 153.4(C-8a), 143.9(C-1), 132.5(C-3), 130.4(C-7), 1.30.0(C-5), 126.9(C-4), 125.9(C- 4a), 122.9(C-6), 122.1(C-2', 6ʹ), 114117. 1 (C-8), 114.2(C-3', 5ʹ), 65.0(C-2), 55.2(C-4-CH2).
MS: m/z 305 [M+H]+
ix) 6-Bromo-2H-chromene-3-(4'-bromophenyl) imine (6c)
Light brown needles; yield 85-87%, mp 137 °C.
IR (KBr): 1638 cm-1(C=N) and 1576 cm-1 (C=C).
UV (MeOH): 355 nm (log ε 4.1) and 272 nm (log ε 4.6).
1H NMR (400 MHz): δ 8.15 (s, CH=N), 7.19-7.32(m, H-5, 7), 7.17(d, J=9.0Hz, H-2', 6ʹ), 6.90(d, J=9.0Hz, H-3', 5ʹ), 6.74(m, H-4, 8), 5.25(s, 2-OCH2).
13C NMR (100.6 MHz): δ 156.8(C-4'), 153.6(CH=N), 152.2(C-8a), 142.0(C-1), 137.8(C-7), 133.5(C-5), 131.3(C-3), 128.4(C-4), 122.1(C- 4a), 120.8(C-2', 6ʹ), 116.1(C-8), 112.7(C-3', 5ʹ), 11l.3(C-6), 63.2(C-2).
MS: m/z 392 [M+H].
x) 6-Methyl-2H-chromene-3-(4'-methylphenyl)imine (6d)
Yellow needles, yield 83-86%, mp l09 °C.
IR (KBr): 1635 cm-1 (C=N) and 1578 cm-1 (C=C).
IR (KBr): 1635 cm-1 (C=N) and 1578 cm-1 (C=C).
1H NMR (400 MHz): δ 8.12(s, CH=N), 7.10(d, J=9.0Hz, H-2',6'), 6.84(d, J=9.0Hz, H-3',5'), 6.74(s, H-4), 6:67-6.78(m, H-7,8), 6.58(d, J=3.0Hz, H-5), 5.16(s, 2-OCH2), 2.35(s,4'-CH3), 2.76(s, 6-CH3).
13C NMR (100.6 MHz): δ 158.5(C-4'), 155.1(CH=N), 154.2(C-6), 149.2(C-8a,144.4(C-1'), 132.6(C-3), 132.2(C-4), 122.5(C-4a), 122.3(C- 2', 6ʹ), 116.6(C-7), 116.4(C-5), 114.4(C-3ʹ, 5ʹ), 112.4(C-8), 65.0(C-2), 55.7(6-CH3), 55.2(4ʹ-CH3).
MS: m/z 265(M+) (85).
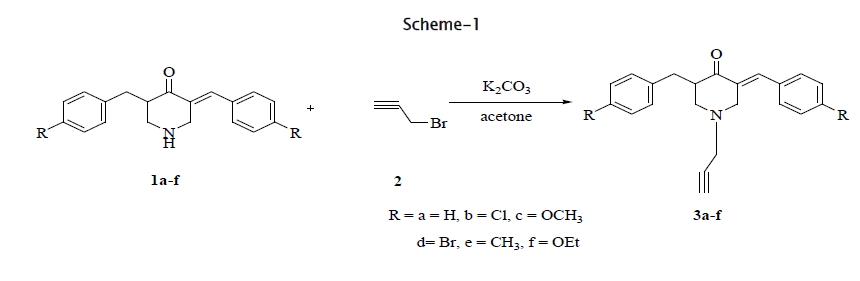
Synthesis of 3, 5 -dibenzylidene-1-prop-2- ynyl-piperidin-4-ones (3a-f).
3,5-Dibenzylidene-piperidin-4-one (1) dissolved in acetone, propargylbromide (2) K2CO3 was added and stirred at room temperature for 8 hours, to give 3,5-dibenzylidene-1-prop'2-ynyl-pip,eridin- 4- one (3a-f). The structure of 3, 5-dibenzylidene-1- prop-2-ynyl-piperidin-4-one characterized from its spectral data. In the IR spectrum 3a, the peak at 2900 (CN), 1650(C=O). The 1H NMR of 3,5-dibenzylidene-1-prop-2-ynyl-pip,eridin-4- one was newly formed triazol ring appeared at around δ7.60 as a singlet and phenyl protons appeared at δ 7.23-7.47 as multiplet. The N-CH2 protons appeared at around δ 3.83, benzylic CH2 protons appeared at around δ 5.50 as singlet.