e-ISSN: 2319-9849
e-ISSN: 2319-9849
Chemistry Department, Yarmouk University, Irbid – Jordan
Received date: 13/06/2015 Accepted date: 19/10/2015 Published date: 22/10/2015
Visit for more related articles at Research & Reviews: Journal of Chemistry
The electrochemical behaviour of stainless steel 302 has been investigated in 0.10 M K2SO4 solution using the impedance technique at several anodic potentials. Stainless steel 302 is passive up to 1300 mV, due to the main formation of Cr2O3. Above this potential stainless steel became active. Faradiac impedances of a heterogeneous chemical reaction and an adsorption process were discussed.
Faraday impedane, Adsorption process, Heyerogeneous chemical reaction, Stainless steel.
Electrochemical impedance spectroscopy (EIS) is widely used [1-4] to study the electrochemical properties of metals and alloys. Physical properties of a passive layer such as polarization resistance (Rp), capacitance (C), and a thickness of oxide layer (d) can be easily determined using the EIS technique. Furthermore, it has been pointed out [5,6] that the impedance of a heterogeneous chemical reaction ZH and of an adsorption process Zad coluld be followed using EIS (Figure 1).
ZH composed of a capacitance of an adsorbed (heterogeneous) speciesn (CH), reaction inhibition resistance, (Rr) and of Warburg diffusion elements (W1) and (W2) while Zad contains the capacitance of an adsorbed material (Cad) Warburg diffusion element (W) and diffusion resistance RD. The main objective of the present study was to investigate the electrochemical behaviour of stainless steeel in 0.10 M K2SO4 solution.
The used stainless steel 302 consisted of iron with 3.42 wt% O, 0.57 wt% Si, 17.91 wt% Cr, 1.9 wt% Mn, 8.0 wt% Ni and 2.16 wt% Cu. An area of 0.78 cm2 of the working electrode was exposed to 100 ml of 0.10 M K2SO4. The working electrode was successively abraded with emery paper up to 1000 grade and then cleaned with acetone. A platinum electrode served as counter electrode while the reference electrode was a saturated calomel electrode (SCE). The used glass cell is described elesewhere [7]. EIS measurements were followed in the frequency range 10-1–105 Hz with an amplitude of 10 mV of using a computerized impedance unit (IM5d produced by Kronach, Germany) after 30 min of anodic polarization at several selected anodic potenials. All patentials were measured against SCE at 25°C.
Current densities for stainless steel in 0.10 M K2SO4 were measured at several potentials in the anodic range 300–1500 mV using the impedance unit. The variation of potential with current density is represented in Figure 2. It is clear from Figure 2 that stainless steel is passive in the potential range 0-1300 mV due to the flow of very small current density values (nano- A and micro ampere scale) and it is active at potentials >1300 mV, as a result of flowing of very high current density values reaching 15.6 mA at 1500 mV.
Two electrochemical equivalent circuit models were found to analyzed the measured EIS spectra for stainless steel in 0.10 M K2SO4 solution (Figure 3).
Models 1 and 2 were used to analyze the measured EIS spectra. Both models contained solution resistance (Rs) resistance of solution between the working and reference electrodes, and Young impedance
Young impedance [6] describes a heterogeneous passive oxide film at the electrode surface and it consists of a capacitance of an oxid film, (C), loss factor (P), which is a measure for surface roughness and time constant (τ).
EIS spectra for stainless steel were measured in newly prepared solution of 0.10 K2SO4 at 400 and 800 mV every 30 min for 210 min. Figure 4 represents the analyzed impedance spectrum for the electrode at 400 mV after 150 min of anodic polarization. Similar EIS spectra were obtained for the rest of the measurements at 400 and 800 mV. The points in Figure 4 are the measured values whereas the solid line connecting between the points is the result of simulation according to model 1 in Figure 3. The simulation was considered to be acceptable when the relative error in the values of impedance and phase angle shift is less than 2% and 0.3°, respectively, using a computerized program for the simulation. According to Figure 4, the impedance line in the frequency range 10-1–104 Hz is straight line with increasing impedance values as frequency decreases. The impedance values at frequencies >104 are independent on frequency, respresenting Rs. The phase angle shift curve shows a plateaue in the middle frequency region, indicating the presence [6] of Young impedance and Warburg diffusion element with 45° phase angle shift.
In order to invesigate the effect of each element composing the electrical circuit, it is possible to change the value of any impedance element, keeping all the other values as obtained from the simulation, by 10%. For example W1 in ZH affect the EIS spectrum in the fequency range 1–103 Hz.
The analyzed impedance values of stainless steel at 400 mV in 0.10 M K2SO4 as a function of time are given in Table 1. According to this table, the values of current densities were very small (nA) and decreased with time. The values of C, describe the capacitance of the main passive oxide layer at the electrode surface, were decreased with increasing time, indicating a growth in the thickness (d) of the formed passive oxide film (mainly Cr2O3) according to equation 1[6]:
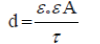 (1)
(1)
| Time min. | Current nA | CmF | P% | t ns | CHmF | RrW | W1 kWs-0.5 | W2 kWs-0.5 |
|---|---|---|---|---|---|---|---|---|
| 60 | 346 | 9.7 | 5.1 | 212 | 7.6 | 90 | 9.1 | 1.1 |
| 90 | 333 | 9.3 | 5.1 | 210 | 7.4 | 95 | 9.2 | 1.1 |
| 120 | 251 | 9.2 | 5.1 | 213 | 7.1 | 100 | 9.3 | 1.2 |
| 150 | 203 | 9.2 | 5.1 | 223 | 6.9 | 101 | 9.5 | 1.2 |
| 180 | 217 | 9.1 | 5.2 | 220 | 6.8 | 100 | 9.5 | 1.2 |
| 210 | 198 | 8.8 | 5.1 | 217 | 6.7 | 205 | 9.2 | 2.3 |
Rs=23.2 Ω
Table 1: Analyzed impedance values for stainless steel in 0.1 M K2SO4 at 400 mV according to model 1, figure 3.
Where ε0, ε and A are the permittivity of free space, dielectric constant of the oxide and the surface area of the electrode, respectively.
The surface roughness of the electrode was not changed as it can be seen from the constant values of P. The values of the time constant (τ) were increased slightly from 285 ns at 60 min to 296 ns at 210 min of anodic polariztion, indicating a small decrease in the conductivity (σ) of the formed oxide film at the electrode surface according to equation 2[6]:
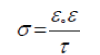 (2)
(2)
Scheme 1 explains the trend varaition of the values of ZH components. With increasing time, the thickness of the adsorbed (due to the interacion between the negative OH- and the positive electrode) species as a result of a decrease in the values of CH . This behaviour is accomained by an increase in the values of W1, W2 and Rr. Due to the anodic oxidation of OH- a concentration gradient of OH- near the electrode appears, so the values of W2 increases. Consequently, the values of W1 of the adsorbed material (H2O molecuels diffuse toward the solution) increase. The values of Rs were kept constant with a value of 23.2 Ω.
Newly prepared soltuin of 0.10 M K2SO4 is slightly basic and the pH of the solution at the end of the experiment was 7.1. The possible mechanisms [8] of anodic oxidation of a basic or slightly acidic aqueous solutions are shown in schemes 1 and 2, respectivly:

Scheme 1 explains the trend varaition of the values of ZH components. With increasing time, the thickness of the adsorbed (due to the interacion between the negative OH- and the positive electrode) species as a result of a decrease in the values of CH. This behaviour is accomained by an increase in the values of W1, W2 and Rr. Due to the anodic oxidation of OH- a concentration gradient of OH- near the electrode appears, so the values of W2 increases. Consequently, the values of W1 of the adsorbed material (H2O molecuels diffuse toward the solution) increase. The values of Rs were kept constant with a value of 23.2 Ω.
It is important to compare between the behaviour of stailess steel at 400 mV and at open circuit potetial (Eoc). The value of Eoc was -210 mV. At Eoc the analyzed impedance values of C, P, τ, CH, W2, and Rs were 19.2 μF, 5.1%, 207 ns, 26.2 μF, 623 Ω and 21.2 Ω respectively. A comparison between the analyzed impedance values obtained at Eoc with those obtained at 400 mV the followings could be obtained:
I. The values of C and τ at Eoc are higher than the values obtained at 400 mV, because the surface of the electrode at Eoc was cleaned and abraded with emery paper.
II. A weak adsorption process (weak interaction between the electrode and ions in solution) occurred at Eoc. As a result CH presents only (with higer vallues than those obtained at 400 mV) and W2 in the analysis. In this case CH is better replaced by Cad.
The analyzed impedance values for stainless steel in 0.10 M K2SO4 at 800 mV, according to model 1 Figure 3, are given in Table 2. The behaviour of stainless steeel at 400 and 800 mV is the same. Upon comparison between the values of similar impedance elements, under the same conditions, in Tables 1 and 2 the following conslusions could be obtained :
| Time min. | Current nA | CmF | P% | τ ns | Cad mF | RrW | W1 KWs-0.5 | W2 kWs-0.5 |
|---|---|---|---|---|---|---|---|---|
| 60 | 192 | 10.0 | 4.7 | 285 | 9.3 | 10 | 6.4 | 0.65 |
| 90 | 124 | 10.0 | 4.7 | 285 | 9.0 | 16 | 66 | 0.68 |
| 120 | 92 | 9.7 | 4.7 | 288 | 8.8 | 20 | 6.6 | 0.69 |
| 150 | 94 | 9.6 | 4.7 | 292 | 8.6 | 20 | 6.6 | 0.70 |
| 180 | 72 | 9.5 | 4.7 | 295 | 8.6 | 24 | 6.7 | 0.73 |
| 210 | 67 | 9.4 | 4.6 | 296 | 8.5 | 30 | 6.8 | 0.75 |
Rs=23.2 Ω
Table 2. Analyzed impedance values for stainless steel in 0.1 M K2SO4 at 800 mV according to model 1, Figure 3.
I. The values of the current densities at 800 mV are higher than the values of the current densities at 400 mV, leading to a higher rate of OH- oxidation. Consequently, the values of C at 800 mV became smaller (due to an increase in the thickness of the oxide layer)
II. Upon increasing anodic potential from 400 to 800 mV more adsorption of OH- occurred, leading to smaller values of CH which reftects a higher values of W1 and W2.
III. Remarkably, the values of Rr at 800 mV are smaller than the values of Rr at 400 mV, indicating a more favorable oxidation process to occur at 800 mV.
Figure 5 represents the EIS spectra for stainless steel in 0.10 M K2SO4 after 30 min of anodic polariztion at 1000, 1100, 1200, 1300, 1400 and 1500 mV with increasing anodic potentials, the curves of impedance and phase angle were shifted to lower values. The values of impedance are independent on frequency at low and high frequency regions and the plateaue at the phase angle curve is completely lost at 1400 and 1500 mV. Results of the analysis of of all measured EIS spectra in the potential range 1000-1500 mV according to model 2 Figure 3, are given in Table 3.
| Potetial mV | Current A | CF | P% | τ ns | Cad μF | W kΩs-0.5 | Rad Ω |
|---|---|---|---|---|---|---|---|
| 1000 | 5.62 m | 939 n | 7.5 | 294 | 21.6 | 25.3 | 350 |
| 1100 | 13.5 m | 617 n | 7.9 | 279 | 22.5 | 21.2 | 365 |
| 1200 | 25.3 m | 600 n | 8.3 | 264 | 30.1 | 18.2 | 280 |
| 1300 | 52.6 m | 595 n | 8.8 | 256 | 47.4 | 13.1 | 178 |
| 1400 | 2.05 m | 5.75 m | 8.8 | 83 | ¾ | ¾ | 212 |
| 1500 | 16.5 m | 28.8 m | 8.9 | 81 | ¾ | ¾ | 187 |
Rs=25 Ω
Table 3. Analyzed Impedance Values for Stainless Steel after 30 min of anodic polarization in 0.10 M K2SO4 according to model 2, Figure 3.
Faradiac impedance of the adsorption process, Zad, mainfests itself clearly in the analysis of the measured EIS spectra in the potential range 1000-1300 mV. Zad decribes simply the adsorption of species present in solution such as OH-, SO42- and H2O.
In the potential range 1000-1300 mV an increase in potential is accompanied by an increase in the values of current densities. The values of C decrease with increasing potential and increasing in the current density values, indicating a growth in the thickness of the oxide layer. This behaviour could be discused as explained before when the potential had been increased from 400 to 800 mV. The % of P increases with increasing potential, indicating an increase in the surface roughness. The conductivity of the formed oxide film increases as the values of τ decrease. As a result of folowing of high current densities, the thickness of the adsorbed layer at the electrode surface increases through an increase in the values of Cad, which is accompained by a decrease in the values of W and Rad. At 1400 and 1500 mV, stainless steell loss completely its passivity through a jump increase in the values of current densities and C as well as a sudden decrease in the values of τ (the electrode bcame well coductive).
Although anodic oxidation of OH- or H2O still occurs (pH of solution is 6.6) in the potential range 1000-1300 mV but the analysis of the measured spectra using model 1 Figure 3, was insatisfactory, probably due to the very high rate of anodic polariztion of OH- or H2O scheme. An attempt was done to combine both models together through series concetion of model 2 Figure 3 with ZH and Rs , the Results of analysis were unacceptable specially under anodic polariztion at potentials less than 1000 mV, where the oxidation process is the rate determining step.
Stainless steel is passive in 0.10 M K2SO4 under anodic polarization up to 1300 mV by vertue of an increase in the thickness of the passive oxide layer. The measured EIS spectra under anodic potentials up to 800 mV could be easily anlayzed using the Faradiac impedance of a heterogenous chemical reaction ZH . The faradiac impedance of an adsorption process Zad could be used for analysis under anodic potentials ≥ 1000 mV.