e-ISSN: 2347-7857 p-ISSN: 2347-7849
e-ISSN: 2347-7857 p-ISSN: 2347-7849
1Department of Pharmaceutics, Academy of Pharmaceutical Sciences, Pariyaram Medical College, Pariyaram, Kannur District, Kerala, India
2Department of Pharmaceutics, Academy of Pharmaceutical Sciences Pariyaram, Kerala, India
3Department of Pharmaceutical Chemistry, Academy of Pharmaceutical Sciences, Pariyaram, Kerala, India
Received date: 07 October 2013 Accepted date: 31 December 2013
Visit for more related articles at Research & Reviews: Journal of Pharmaceutics and Nanotechnology
The mucoadhesive buccal film of Lisinopril were prepared by using various polymers like HPMC K4M, sodium CMC, PVP K30, eudragit RL 100, carbopol 934 by solvent casting method and evaluated for physical appearance, thickness, weight uniformity, folding endurance ,percentage swelling index, percentage moisture content, drug diffusion studies and drug content estimation.Among all formulation, buccal film prepared with 4% HPMC K4M,0.5% PVP K30 1% Sodium CMC and 5% tween 80 as surfactant exhibited better drug release, drug content estimation and maximum percentage of swelling index. The drug release could be extended up to 8 hr and a drug release of 98.41% was observed. FTIR studies revealed that drug and excipients are compatible. Stability study of selected optimised formulations were done as per ICH guidelines for 1 month, which revealed that no significant change with respect to the evaluations conducted before stability charging.
Lisinopril, mucoadhesive buccal film, HPMC K4M, , eudragit RL100, sodium CMC, PVP K30, Carbopol 934
Amongst the various routes of drug delivery, oral route is perhaps the most preferred to the patient .However peroral administration of drugs has been associated with hepatic first pass metabolism and enzymatic degradation within the GI tract that prohibits oral administration of certain classes of drugs especially peptides and proteins [1]. Buccal delivery offer direct access to the systemic circulation through the external jugular vein thus bypassing the drugs from the hepatic first pass metabolism. This may lead to higher bioavailability of such drugs [2].
Buccal drug delivery involves the administration of desired drug through the buccal mucosal membrane, which forms the lining of the oral cavity. This route is useful for mucosal (local effect) and transmucosal (systemic effect) drug administration. In the first case, the aim is to achieve a site-specific release of the drug on the mucosa for local action, whereas the second case involves drug absorption through the mucosal barrier to reach the systemic circulation [3]. The buccal mucosa permits a prolonged retention of a dosage form especially with the use of mucoadhesive polymers without much interference in activities such as speech or mastication unlike the sublingual route [4].
Lisinopril is an orally active non sulfhydryl angiotensin converting enzyme (ACE) inhibitor indicated for the treatment of patients with hypertension, heart failure or with acute myocardial infarction. Lisinopril on oral administration undergoes extensive metabolism in the liver resulting into very poor (approximately 25%) bioavailability [5]. In order to improve its bioavailability, efficacy and to minimize the side effects associated with oral administration, mucoadhesive buccal films of Lisinopril using HPMC K4M alone and in combonation with Na CMC,PVP K30,Eudragit RL100 and carbopol 934 were prepared by solvent casting technique in the present investigation.
Lisinopril was a gift sample from Hetero drugs Ltd.Hyderabad. HPMC(K4M) ,eudragit RL100 and carbopol 934 were obtained from Balaji chemicals,Gujarat.Na CMC was obtained from Nice chemicals Pvt Ltd,cochin and PVP K30 was obtained from Loba chemi Pvt Ltd,Mumbai.Other chemicals used were of analytical grade.
Preparation of buccal films containing Lisinopril
Buccal films of Lisinopril were prepared by solvent casting method. The specified ratios of polymer solutions were prepared by dissolving required amount of polymers ( HPMC K4M alone and in combination different other polymers) in distilled water and stirred by using a mechanical stirrer at 50 rpm for 2hrs and the values are given in the Table No.1. The resulting polymer solution was plasticized using glycerin (6% of total volume). 10 ml of the above solution was added with calculated amount of Lisinopril. This solution was sonicated for 15 min and kept overnight to remove air bubbles and poured in to a glass mould having a surface area of 40 cm2. It was dried in room temperature and the dried film were cut into 2×2 cm and wrapped in aluminium foil and kept in a desiccator. The same procedure was followed to fabricate all baccal films as per Table No.1
Evaluations of developed film [8]
Physical appearance
All the films were visually inspected for colour, transparency and smoothness.
Folding endurance
Strip of prepared film (2 × 2cm) was folded repeatedly at the same place till it broke. The number of times the film could be folded at the place without breaking or cracking is equal to the value of folding endurance [5].
Thickness (mm)
Films of (2×2 cm) were cut and thickness of films were measured using micrometer screw gauge with a least count of 0.01 mm at five different spots of the films and average was taken.
Weight variation
Weight variation was observed in films of 2×2 cm . Ten films were weighed individually and average was taken.
Surface pH
Buccal films were left to swell for 1 hour on the surface of 2% agar plate, it was allowed to stand until it is solidified to form a gel at room temperature. The surface pH was measured by means of pH paper placed on the surface of the swollen patch [7].
Tensile strength (Kg/cm2)
The instrument used to measure the tensile strength designed in our laboratory especially for this project work. The instrument is a modification of chemical balance used in normal laboratory showed in the Figure No.1. One pan of the balance was replaced with one metallic plate having a hook for attaching the film. The equilibrium of the balance was adjusted by adding weight to the pan of balance. The instrument was modified in such a way that the film can be fixed up between two hooks of horizontal beams to hold the test film. A film of 2.5 cm length was attached to one side hook of the balance and the other side hook was attached to plate fixed up to the pan as shown in figure.
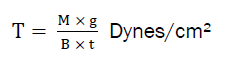
T= force at break/ initial cross-sectional area of sample.
Where,
M = mass in grams
g = acceleration due to gravity 980 cm/sec²
B = breadth of the specimen in cm
t = thickness of sample in cm.
Swelling index [6]
Buccal film of 2 ×2cm area from each formulation was taken. Initial weight of the film was taken by using single pan balance (w1gm) and it was placed in a petri dish containing 50 ml of water. After definite interval film was removed and blotted with filter paper and weighed again (w2gm).
The swelling index was calculated from the formula,
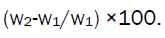
Where w2 =wet weight of the film
W1=dry weight of the film
% Moisture content
The buccal films were weighed accurately and kept in desiccators containing anhydrous calcium chloride. After three days, the films were taken out and weighed.
The %moisture content was determined by the formula,
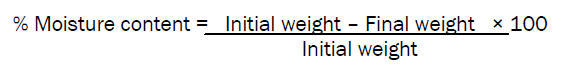
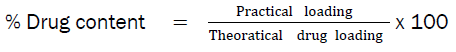
Drug content estimation
Prepared buccal film was dissolved in 100ml PBS of pH 6.8 using a magnetic stirrer for 12 hours and then sonicated for 30 minutes. The solution was centrifuged and then filtered. The drug content determination was done by using UV spectroscopy at 256.2 nm.
In vitro drug release study
In vitro diffusion study was performed by using modified Franz diffusion cell across cellophane membrane. Films of dimension 2x2cm were placed on the membrane, which was placed between donor and receptor compartment of Franz diffusion cell. Cellophane membrane was brought in contact with PBS of pH 6.8 filled in receptor compartment. Temperature was maintained at 370C with stirring at 50 rpm using magnetic beed stirrer. 1ml of sample was withdrawn from receptor compartment at pre-determined interval and was replaced with fresh PBS of pH 6.8. With suitable dilution, samples were measured for absorbance at 256.2nm using UV visible spectrophotometer.
Drug –Polymer interaction studies
The FT-IR studies were carried out for pure drug, pure polymer and mixture of drug-polymer to confirm the interactions. The study confirmed that the test sample was Lisinopril .After spectral comparison it was confirmed that no incompatibility reaction took place between drug and excipient.
Surface of all developed films were smooth, films were flexible with optimum elastic property. The results of physical characteristics were satisfactory for buccal film.
The folding endurance of all the films was found to be above 300. All the films, irrespective of polymers used, showed good folding endurance, thereby ensuring good flexibility.
The thickness of the film F1 and F2 with 3% HPMC was found to be 0.082 and 0.087 respectively. A slight increase in the thickness of the films were observed for all other formulation ( F3-F12) containing 4% HPMC. In all cases, calculated standard deviation values are low which indicates that proposed films were uniform in thickness.
The average weight obtained for the developed films was ranging between 28.66-33.92 mg .No significant variation in the average weight was observed for the developed films. The lowest average weight was found to be with the film containing 3% HPMC and the highest average weight was with film containing 4% HPMC and 2 % sodium CMC. The obtained observations indicated that as the concentration of polymers increased, the average weight of the film also increased.
The pH at mucosal surface is approximately 6.8. The pH between 6.6-6.8 of the developed films indicated that the films may be safe enough for the regular application in the mucosal region.
The percentage swelling index of the films were in between 29.34-37.30%.Formulation F2 had more swelling index than F1,the result indicated that as the concentration of PVP increases percentage of swelling index also increases. But when there is addition of carbopol, swelling index was found to be decreased.
In the case of F9, F10, F11 and F12, the percentage swelling index were found to be almost equal. The value indicated that the addition of surfactant had no effect on the percentage swelling index.
Moisture content calculated for developed films were ranging between 1.2-1.5. No subsequent difference was found between the patches in terms of moisture content
Tensile strength value of developed formulations was in between 2.47-2.89 kg/cm2.
Percentage drug content for all the patches was in between 93-97%. It was observed from the drug content data that there was no significance difference in uniformity of the drug content
In vitro diffusion study was carried out for 8 hour duration with fixed sampling intervals. The release of drug from the matrix was dependent on the nature and concentration of the polymers used. All the results obtained were shown in table and represented graphically.Based on in vitro drug release, formulation F12 with 4% HPMC K4M, 0.5% PVP K30, 1% Sodium CMC and 5% Tween80 exhibited a extended drug release of 98.41% in 8 hours.F12 was selected as optimized formulation.
In the present study it can be concluded that;
• FTIR studies revealed that there is no incompatability or interaction between Lisinopril and excipients.
• Formulated buccal films gives satisfactory film characteristics like physical appearance, surface texture, weight uniformity, thickness uniformity, folding endurance, surface pH, percentage swelling index, percentage moisture uptake, drug content uniformity, in-vitro drug release. The low values for standard deviation for average weight ,thickness, surface pH, percentage swelling index, percentage moisture uptake, in vitro drug release and drug content indicated uniformity within the batches.
• Based on in vitro drug release, formulation F12 with 4% HPMC K4M, 0.5% PVP K30, 1% Sodium CMC and 5% Tween80 exhibited a extended drug release of 98.41% in 8 hours.
• The optimized formulation followed zero order kinetics.
• Short term stability studies of optimized formulation as per ICH guidelines indicated that there is no significant change in physical appearance, drug content determination and in vitro drug release.
So finally it can be concluded that buccal films of Lisinopril could provide sustained buccal delivery for prolonged period. A further clinical investigation has to be conducted to establish the safety and efficacy of the developed formulation.