e-ISSN: 2321-6190 p-ISSN: 2347-2294
e-ISSN: 2321-6190 p-ISSN: 2347-2294
Department of Animal and Environmental Biology Faculty of Life Sciences,University of Benin, Benin City, Nigeria.
Received Date: 15/09/2013; Revised Date: 10/10/2013; Accepted Date: 12/10/2013
Visit for more related articles at Research & Reviews: Journal of Zoological Sciences
The paper examines aspects of the reproductive biology of Synodontis schall from Jamieson River, Nigeria with emphasis on the histology of the gonads. Sampling was carried out between October 2010 and October 2011 and 623 specimens of Synodontis schall were collected using cast and gill nets. In the laboratory, specimens were measured for total length (cm), standard length (cm), weighed (g) and dissected. Standard techniques for sex ratio, gonadal maturation, size frequency distribution of ovarian oocytes and fecundity were used in analysis. Linear regression technique was used to determine the relationship between fecundity and fish length, body weight and ovary weight. The gonads were observed for morphological and histological changes, and the results used to classify their different developmental stages and maturity into five successive stages-Immature, Recovery, Maturing, Ripe and Spawning. The total lengths of ripe specimens range from 19.40cm to 26.20cm with body weights of 73.62gm to 252.15gm and ovary weights of 2.30gm to 28.41gm. Absolute fecundity ranged from 1530 to 13,965 eggs with a mean of 6080 eggs. Gonadosomatic index (GSI) increased with increase in the stage of gonad development for each sex. GSI values were lower in males than females of corresponding stage of development. Fecundity-body weight and fecundity-ovary weight logarithmic relationships were more highly correlated and significant than the fecundity-total length relationship. There is evidence that the female spawns once annually. Results of the above investigation will ensure the conservation of S. schall in Jamieson River, as well as its full utilization as a sustainable resource.
Synodontis schall, gonadal maturation, fecundity, Jamieson River.
Synodontis is a common mochokid genus in many lakes and rivers in tropical Africa [1]. In Nigeria, there are about 18 species and these are of great commercial importance [2,3,4].
Synodontis schall is widely distributed and abundant in the Jamieson River, a tributary of the coastal Benin River in the Niger-delta. They are tasty, much cherished by the local riverine inhabitants and supports a thriving fisheries.
Biological studies of S. schall have been reported [1,4,5,6,7,8]. However, only few reports exist on the aspects of its reproduction. The morphology of the gonads in the reproductive cycle of S. schall from Asa lake, Ilorin Nigeria has been studied [9,10]. Reproductive biology is an important branch of fishery science and it is useful in fish culture and management. The ultimate aim of fisheries management is to attain sustainable exploitation of fisheries resources and this requires a proper understanding of the population dynamics of the fish stock. Reproductive biology is one of the major factors influencing the dynamics of a given population.
The present study was therefore undertaken with a view to contributing to the reproductive biology of S. schall in Nigeria, with emphasis on the histology of its gonads which clearly assist in separating the different stages of gonadal development.
The study was carried out in the Jamieson River (5°41’-5°58’E; 5°54’-6°08’N), one of the two confluent tributaries of Benin River (Fig. 1). It takes its origin from Ugboko-Niro and flows in a south-westerly direction, 70 km, to Sapele, where it empties into the Benin River, which discharges into the Atlantic Ocean at the Bight of Benin.
Jamieson River lies in an area with a tropical rainforest climate. Two main seasons prevail; the wet (May to October) and dry season from November to April the following year. The river flows all year round with the highest level and discharges during the flood period (July-November). The river is subjected to tidal inundation from the Benin River at the Sapele-Sakponba stretch. The fringing plants consists mainly of Cyrtosperma senegalense (Schott) Egnl., Lonchocarpus griffonianus Dunn, Anthocleista vogelii Planch, Pandanus candelabrum P. Beauv and Crinum jagus Thomps.
Monthly samples of Synodontis schall were collected from four locations in the river from October 2010 and October 2011. The fish samples were obtained from hired fishermen who carried out fishing between 0730-1200h during the day and 0200-0400h in the night. The principal gears employed in all the sampling zones were cast nets (35-55 mm stretched mesh size) and gill nets (20-70 mm stretched mesh size). Fish samples caught were transported in an ice box from the fishing site to the laboratory. Routine measurements of length (standard and total) were taken to the nearest 0.1 cm and specimens were weighed to the nearest 0.1 g.
The fish samples were dissected, sexed and the state of the gonads were recorded [11]. Ovaries and testes were detached and weighed to the nearest 0.01g. The data on the body and gonad weights were used to compute the gonadosomatic index (GSI) [12].
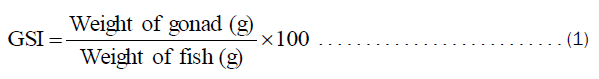
Immature ovaries and all stages of testes were fixed in Bouin’s fluid. For specimens with maturing to mature ovaries, one of the ovaries was fixed in Bouin’s fluid and the other in Gilson’s fluid. All gonads fixed in Bouin’s fluid were later processed, embedded in paraffin, sectioned at 4-6μm, stained with haematoxylin and eosin and used for histological evaluation [13].
Ovaries preserved in Gilson’s fluid, had their eggs separated from the ovarian tissues by frequent vigorous agitation of the specimen bottles after a week. The eggs were then cleaned thoroughly by rinsing with the fixative three to four times and the number of eggs in each pair of ovaries was determined by direct enumeration. Size frequency distribution of intraovarian oocytes was determined by measuring the diameter of one hundred oocytes taken at random from anterior, middle and posterior region of five ripe female specimens [14]. The egg diameters were measured with a calibrated micrometer mounted in the eye-piece of a binocular microscope [15].
The relationship between fecundity and fish length, fish weight and ovary weight were determined by linear regression technique. The best predictive equation was computed as logarithm transformation of the equation.
F = aXb , i.e. Log F =log a + b log X [16].
Where;
F = Fecundity; X = Length/weight; a = Regression constant; b = Regression coefficient
The data obtained in this study were analyzed with the computer using the SPSS package version 16.0. The analysis carried out include:
1. Chi-square test, performed on monthly and overall sex ratios of the population;
2. One way analysis of variance (ANOVA), carried out to test for significant differences and
3. Regression analysis, used to test for linear relationships [16].
Sex Ratio
A total of 265 males and 358 females were recorded giving a sex ratio of 1:1.35 (m:f). In the months of October, 2010, January, February, April and May 2011, significantly higher proportion of females were observed (P<0.05); while in all other months almost equal numbers of males and females (P>0.05) were recorded (Table 1). The overall sex ratio of the population, 1:1.35 differed significantly (P<0.001) from the expected 1:1 ratio.
Maturity Stages
The description of the maturity stages of male and female gonads based on morphological and histological appearances are shown in Tables 2 and 3. Five maturation stages (I, II, III, IV and V) were identified for males and females. Stage VI (spent) gonads were not observed in both sexes.
Figure 2:Photomicrographs showing changes in testes of Synodontis schall (A & B) Transverse section of the immature and maturing testes showing Spermatogonia (SP) and clusters of Primary and Secondary Spermatocytes (PS & SS) with a few Spermatids (S). (C & D) Ripe and Spawning testes showing active spermatogenesis. Sperm ducts distended with Spermatozoa (SZ). Mag. X400 in A, B, D & D.
Figure 3:Photomicrographs showing changes in the ovary of Synodontis schall. (A & B) Transverse section of immature and recovery ovaries showing Oogonia (O) (C) The maturing ovary with Primary Vitellogenic Oocyte (PVO), showing increase in size and accumulation of yolk granules (Yg) droplets. (D) The Ripe ovary with Secondary Vitellogenic Oocytes (SVO) and Post-Vitellogenic Oocytes (PsVO). (E) The Spawning ovary with hyaline Post Vitellogenic Oocytes (PsVO) ready for spawning. Mag X100 in A, D & E; X160 in B and X200 in C.
Gonado-Somatic Index (GSI)
Gonad stages I to V were used in estimating the GSI from the relationship
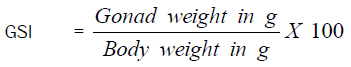
The results are presented in Table 4.
The table shows that the GSI increased with the increase in the stage of gonad development for each sex (mean of 0.07 in stage I to 1.39 in stage V for male, and 0.17 in stage I to 9.58 in stage V for female). It also shows that GSI for the males were always lower than those of the females of corresponding stage of development. For example, while the gravid male testis (stage IV) constitute on the average 1% of the body weight, gravid ovary form averagely more than 3.3% of the body weight.
Diameter of Ova
The ova diameter varied from 0.70 to 1.60mm. The eggs were ovoid in shape, light yellow and were of almost uniform diameter, indicating that they developed equally in all parts of the ovary and the ova matured simultaneously in both the lobes irrespective of their locations in the ovary.
Fig. 4 shows the size frequency of distribution of intra-ovarian oocystes of five randomly selected ovaries in stage V. The modal egg diameter was least (1.0 x 10-1 mm) in specimen B with least total length of 19.80cm, while specimen C with total length of 21.70cm had modal egg diameter of 1.4 x 10-1 mm. The histograms for each of the five specimens show a unimodal peak, classifying the species as a total spawner.
Fecundity
The fecundity of 81 ripe females of Synodontis schall from Jamieson River ranged from 1530-13,965 eggs with a mean of 6080 eggs. The total lengths of these ripe specimens range from 19.40cm to 26.20cm with body weights of 73.62gm to 252.15gm and ovary weights of 2.30gm to 28.41gm. Maximum fecundity was recorded from a fish measuring 21.70gm in total length and 138.69gm in body weight and the minimum, from a fish measuring 24.80gm in total length and 131.71gm in body weight.
Fecundity was plotted against total length, body weight and ovary weight. It was observed that the fecundity increased with the increase in body weight and ovary weight of the fish. The equations of regression coefficient between total length (TL), body weight (BW) and ovary weight (OW) versus fecundity (F) are given below. All these relationships have been shown graphically in Figs. 5, 6 and 7.
LogF = 2.300+1.044 LogTL (r = 0.139)
LogF = 1.921+0.845 LogBW (r = 0.374)
LogF = 2.897+0.905 LogOW (r = 0.934)
In this study, the sex ratio of 1:1.35 in favour of females is similar to that reported for Synodontis schall in Asa Lake, Ilorin [10]. However, Imevbore observed almost equal proportion of male and female for S. gambiensis (I:I), Hemisyonodontis membraceous (I:I), S. budgetti (1:050) and S. violaceous (1:077) from River Niger [17]. According to Araoye, a higher female population can reduce competition among males for courtship activities with the females during the season of reproduction [10].
The testicular and ovarian cycle of Synodontis schall in this investigation can be divided into five stages-Immature, Recovery, Maturing, Ripe and Spawning. All the gonad stages showed visible morphological and histological changes during development. These changes, which occurred during the maturation processes, conformed to the general pattern of development of the gonads in most teleosts [18,19,20].
In the juvenile stage, the ovary consists of very small spherical oogonia and a few primary oocytes, constituting the first growth phase or previtellogenesis. The recovery and maturing stages constitute the second growth phase or vitellogenesis; which is characterised by rapid growth. During this period oocytes increase rapidly in size due to accumulation of yolk materials in the oocytes cytoplasm. Next is the ripe and spawning stages which consist mainly of secondary vitellogenic and post-vitellogenic oocytes. This is the period of oocyte maturation, when oocytes had accumulated enough yolk, become matured and ripe and ready for ovulation and spawning. Similar observations were reported for Synodontis schall from Asa lake, Ilorin [10], African lungfish (Protopterus annectens) from River Niger [21] and Tilapia mariae and Chromidotilapia guentheri from Jamieson River [22,23]. However, Araoye, who worked on same species as in the present study, only examined the morphological features of the gonads [10]. The use of histological descriptions is important in clearly separating the different maturity stages and enables one to access correctly the level of reproductive activity (fertility) of a measured fish specimen.
Male and female specimens of S. Schall were observed to start breeding activities at the onset of the rains and continue during the rainy season (May-September). Spawning occurred later during the flood between July-September. This result is similar to that reported for same species [4,9,10]. Halim and Guma’a reported that S. schall spawns from July to September and noted that females are ripe at the early age of I [9]. Araoye noted that spawning was between June to September with peak period occurring in July and August when the lake became flooded due to the rains [10]. Olatunde observed that spawning in S. schall may continue till the early dry season in November because of a large number of specimens with spent gonads caught at this period [4]. In the present study, however, no spent specimen of S. schall was encountered.
The gonado-somatic index (GSI) for males was lower than that of females of corresponding stage of gonadal development. Similar observations have been reported [22,24]. It has also been observed that for African species, the GSI is generally higher for females than for males [25].
Absolute fecundity of S. schall ranged from 1530 to 13,965 eggs with a mean of 6080 eggs. The estimate of fecundity in the present study was much lower than the estimates reported for same species (10,000-90,000 eggs) [9] and (7910 to 64, 450 eggs) [10]. Although, the ovary weights of the specimens (6.30-43.75g) from Asa lake were observed to be larger in size than those of the present study (2.30-28.41gm). However, similar fecundity estimates were reported for S. batensoda (6850-20,400 eggs) and S. nigrita (952-11,400 eggs) from Lake Kainji [26]. The change in fecundity estimation could be due to different environmental conditions in which these different populations live. In general, compared to most freshwater fishes, Willoughby, noted that members of-the genus Synodontis have always exhibited high fecundity and this can be attributed to the small size of their eggs [26]. According to Olatunde, high fecundity is an advantage because of the continued existence of fish which depends on the number of eggs hatched and their survival to adult stage [4].
Results from this investigation, showed that fecundity-body weight and fecundity-ovary weight logarithmic relationships were more highly correlated and significant than the fecundity-total length relationship. Similar observation was reported by Araoye for S. Schall [10], but Halim and Guma'a noted a high correlation between fecundity and body length for same species from the White Nile near Khartoum [9]. The positive correlation between fecundity-body weight and fecundity-ovary weight may be attributed to the high fecundity characteristic of members of the genus Synodontis [10].
All the ova (100 from each ovary) were found to be ovoid and almost uniform in diameter. This indicates that the eggs shed in a season developed simultaneously and were shed in a single batch, classifying S. schall as a total spawner.