1Materials Science Centre, Indian Institute of Technology, Kharagpur, WB-721302, India
2School of Medical Science and Technology, Indian Institute of Technology, Kharagpur, WB-721302, India
Received date: 21/03/2015; Accepted date: 12/07/2015; Published date: 17/07/2015
Visit for more related articles at Research & Reviews: Journal of Medicinal & Organic Chemistry
In the present investigation the surface of low density polyethylene (LDPE) films have been functionalized with acrylamide (AAm) using solution grafting technique. The surface chemistry, structure, roughness and wettability of modified LDPE film have been studied using ATR–FTIR spectrophotometry, scanning electron microscopy (SEM), atomic force microscopy (AFM), contact angle measurement, respectively. Subsequently, the films were screened on their fouling behaviour by cell adhesion and proliferation of HaCaT cells with different grafting times. The degree of grafting of AAm was assessed by means of carbonyl index (CI) in the wave–number of 1672–1633/719–721 cm-1 in the FTIR spectra. The grafted film was found to be hydrophilic in nature as seen from its water contact angle (45° ± 2°). The Nano indentation study revealed that the surface mechanical property of the film has been slightly changed after AAm grafting. The grafted film was found to be non–toxic and biocompatible with HaCaT cells as confirmed by the Alamar blue assay. Thus, it is understood that the AAm grafted LDPE film is a potential candidate for biomedical applications and also useful for other polar polymer surface coatings.
LDPE, Solution grafting, Surface functionalization, Biocompatibility, Cell adhesion
Surface functionalization is one of the successful techniques to attach hydrophilic groups on polyolefin which are basically hydrophobic in nature with low surface energy. For biomedical application, it is necessary that the designed polymeric device must be biocompatible with the cellular materials [1]. Surface functionality and topography play an important role in enhancing the cell viability index and bio-functionality of the polymeric materials. It has been observed by the researchers that the adhesion and proliferation of cells on polymeric materials depend on surface characteristics such as hydrophilicity or hydrophobicity, wettability, surface charge, roughness, etc.
Low density polyethylene (LDPE) is a promising polymer for biomedical applications due to its excellent chemical resistance, high impact strength, light weight and high flexibility. Many medical devices e.g. meshes, catheters and artificial joints have been developed in the pure form of LDPE [2,3]. On the other hand, it has several limitations because of low surface energy and nonpolar nature. Therefore, surface modification of LDPE is one of the strategies to improve the surface wettability, adhesion and biocompatibility via introducing polar entities and changing surface topography [4]. A variety of techniques have been applied such as plasma treatment [5,6], chemical and physical etching [7-11], corona discharge and surface grafting to enhance the surface characteristics of LDPE [12,13]. Among these techniques, surface grafting with polar vinyl monomer is a prominent method. Different vinyl monomers such as acrylic acid (AA) [14,15], 2-hydroxymethyl methacrylate [16], vinyl acetate (VA), acrylamide (AAm) and 1-vinyl-2- pyrrolidone (NVP) [17], methacrylic acid [18], vinyl triethoxysilane and cyclodextrin [19,20] have been reported for LDPE surface grafting. These monomers were grafted using different radiation techniques such as β and γ-irradiation, UV-irradiation, photo and plasma induction. For instance, Ishihara et al. reported photoinduced graft polymerization of 2-methacryloyloxyethyl on polyethylene membrane and demonstrated the blood cell adhesion on the modified membrane surface [21]. Loh et al. investigated the structural properties of the UV light induced surface grafted AAm, AA and N, N-dimethylaminoethyl methacrylate onto polyethylene, poly(ethylene terpathalate) and polystyrene [22]. Wirsen et al. explored the graft polymerization of AAm onto linear low-density polyethylene film using electron beam pre-irradiation [23]. Kubota et al. used N-isopropylacrylamide (NIPAM) for grafting onto LDPE film by photo-irradiation [24]. Chun et al. reported graft copolymerization of mixtures of AA and AAm onto LDPE film surface [25]. Gupta et al. described the radiation grafting of AAm into polyethylene films and studied the thermal properties of grafted film [26]. Fayek et al. reported AAm graft copolymerization onto LDPE surface using γ-irradiation technique [27]. Dessouki et al. demonstrated post radiation grafting of VA onto LDPE film [28]. Queiroz et al. reported radiation-induced AA grafting onto LDPE [29].
These radiation based grafting may change the physical properties of polymeric materials, also require very high instrument cost and moreover these are not user-friendly. Thus, the aim of the present work is to prepare biocompatible LDPE through solution grafting of a hydrophilic polymer onto the surface of the LDPE film without affecting the bulk properties of polymer. Further AAm was grafted to the LDPE surface through solution grafting method that involved first the surface oxidation followed by grafting of polyacrylamide. Surface of the grafted LDPE was characterized using ATR-FTIR spectroscopy and contact angle measurement. Surface topography was analyzed using scanning electron microscopy (SEM) and atomic force microscopy (AFM). The effect of the grafting on mechanical strength was measured using universal testing machine (UTM). Next human keratinocytes cell (HaCaT cells) was grown on polyacrylamide grafted LDPE film to see the effects of polyacrylamide grafting on cell adhesion and proliferation.
Materials
Commercial low density polyethylene (LDPE) in the form of beads, (general grade I005FY20, Melt flow index (MFI) 0.5 g/10min, density 0.92 g/cm3) was purchased from Reliance Industries Limited, India. AAm of synthesis grade was purchased from Spectro chem Pvt. Ltd. India and was used as received. Ammonium persulfate (APS), ferrous ammonium sulphate and nitric acid (70%) were purchased from Merck Pvt., Ltd. India. Initiator, ceric ammonium nitrate (CAN) was supplied by Qualigens Fine Chemicals, India. Milli-Q water was used in all experiments. Grade-1 (maximum impurity 10 ppm) nitrogen gas was used for purging purpose. Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), HaCaT cells from NCCS Pune and Alamar blue from Life Technologies USA were procured. Multi-well tissue culture plates were procured from Nunc, Denmark.
Preparation of AAm grafted LDPE film (AAm-g-LDPE) and characterization
AAm was grafted onto LDPE film via solution grafting technique in the presence of APS and CAN. In reaction process deionized water was used as a solvent. First, the LDPE beads were cleaned with vigorous stirring in acetone and then with distilled water and dried in oven at 50°C for 2 h. LDPE films (100 μm-thick) were prepared by compression moulding of cleaned LDPE beads. Beads were pressed in between Teflon sheets under 1 MPa pressure at 130ºC for 15 min without using any mould releasing agent.
In the next step, the grafting reaction was carried out under nitrogen atmosphere in a 250 mL three necked round bottom flask (RBF) containing 100 mL de-ionized water and equipped with a reflux condenser, thermometer, a stirrer, and gas inlet outlet system. In this reaction setup 4 g LDPE specimen having 5 cm × 1 cm dimension was placed. Freshly prepared 10 mL of 0.025 M APS solution was used as a hydroxylating agent for the LDPE [30,31]. Nitrogen gas was purged for 10 min to remove dissolved oxygen from water and the system was left for 10 h at 60 ºC. After 10 h, a freshly prepared 5 mL solution of 0.36 mg CAN in 0.1 N nitric acid was added and stirred for 30 min and the setup was left for 2 h. Turbidity was observed within the first 30 min. It was assumed that in the presence of CAN, hydroxyl groups decomposed and generated alkoxy radicals which initiated the grafting on the surface of LDPE [30]. The flow of nitrogen gas was kept continuous through the reaction mixture and then different amounts of AAm were added to reaction mixture drop-wise. Ferrous ammonium sulfate (Mohr's salt) as inhibitor was added into reaction chamber to avoid homopolymerization of the monomer and it increased the grafting yield [30,32]. The grafting reaction was carried out for different time intervals at 70º C with stirring (500 RPM). The different steps of grafting are shown in Scheme 1 a-1c. In the initiation step (Scheme 1a) APS homolytically decomposed and formed hydroxyl radicals. The resultant radicals (˙OH) were assumed to interact with the LDPE, producing alkyl radicals via hydrogen abstraction in Scheme 1b. Next these alkyl radicals interact with hydroxyl radicals to form hydroxylated LDPE (R-OH). After isolation of the hydroxylated LDPE (R-OH), decomposition of hydroxyl groups at the surface in the presence of CAN generated alkoxy radicals (R-O˙) which initiated the grafting in Scheme 1c.
At the end of grafting reaction, the films were washed first with water/methanol (1:1) solvent mixture to extract the residual monomer and the homopolymer occluded on the film surface. Grafted films were dried in vacuum oven at 60ºC till constant weight was obtained and stored for further characterization.
Surface chemical analysis
The introduction of functional entities on the LDPE surface was analysed using ATR-FTIR spectroscope (Thermo Nicolet, NEXUS 870 IR) in the range 4000 to 500 cm-1.
Grafting yield (%) analysis
To determine the grafting yield, the mass of AAm grafted on the LDPE film was measured using micro balance (Model- CPA26P, Sartorious, Germany,). The amount of AAm grafting onto LDPE films was calculated as follows:
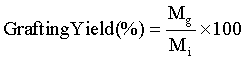 (1)
(1)
where Mg and Mi are the masses of the grafted AAm onto LDPE and initially used AAm, respectively.
Surface morphology study
The surface morphology was qualitatively investigated using a scanning electron microscope (SEM, JEOL JSM-5800, Japan) using secondary electron beam. SEM micrographs were utilized to examine the physical changes caused by the solution grafting technique of AAm on the surface of LDPE.
A Nanonics Multiview 1000 TM (Israel) SPM system was used to determine the surface topography and roughness of the AAm grafted LDPE samples. For the atomic force microscopy (AFM) analysis, the cantilever tip had diameter of 20 nm and spring of force constant 40 N/m was used. The root-mean-squared roughness (Rrms) and the maximum height for the topographic profile were measured.
Surface hydrophilicity measurement
Contact angle meter (S. E. O. Co. Ltd, Model-300, Korea) was used for the measurement of contact angle (using sessile drop method) of pristine LDPE and acrylamide grafted LDPE film, where distilled water was used as the probe liquid. For consistency, the contact angle readings were recorded just after 20 sec from the time of placing the liquid droplet (1-2 μL) on the substrate surface. Five different readings were recorded and the average value of the contact angle was taken for each sample.
Mechanical property analysis
The tensile strength of the AAm grafted LDPE films were measured according to the ASTM, D-638 (with a cross head speed of 5 mm/min, 500 N, at room temperature). All the samples were tested using universal testing machine (HOUNSFIELD H10KS UTM) with gauge length of 20 mm. For each sample five measurements were performed and the average data were recorded.
Nanomechanical property analysis
To determine the effect of AAm grafting on the top layer of film was analysed by nanomechanical property study. The nanomechanical property measurements of the pristine LDPE and grafted LDPE were carried out using Nano-Triboindenter (TI 950 TriboIndenter, Hysitron Inc., USA) at room temperature. Tests were performed in the quasi-static mode and the maximum load was applied 400 μN (for ten different points) at a rate of 10 μN/s for each test. The load was held for 20 s (creep time) to avoid the ‘nose problem’ and then unloaded to 90% of the maximum load at the same rate. The indenter displacement is obtained as a force displacement curve during loading and unloading conditions, which were recorded for data analysis. Berkovich (a threesided pyramidal geometry) diamond tip is used in all measurements.
Cell adhesion study
Indirect cell counting method was employed for cell adhesion test on samples. Briefly, immortal human keratinocytes cell line (HaCaT cells) was cultured in DMEM + 10% FBS complete medium in an humidified incubator set to deliver 37°C temperature and 5% CO2 environment. 0.5 × 105 cells were seeded onto 1 cm × 1 cm polymeric substrates. A same number of cells were also seeded onto 1 cm × 1 cm cut-outs of polystyrene coated tissue culture plate, which served as a control. The cell seeded sheets were placed in cell non-adhesive culture plates followed by their transfer to the incubator. The plates were incubated for different time intervals wherein unadhered cells were collected and counted under a phase-contrast microscope with the help of a Neuber’s hemocytometer. The numbers of unadhered cells were subtracted from the original number of seeded cells in order to obtain the number of adhered cells (Equation 2).
Nadhered cells = Nseeded cells - Nunadhered cells
HaCaT cell suspension was applied on different polymeric substrates (1 cm × 1 cm size) and incubated in humidified CO2 incubator (37°C, 5% CO2) for 3 h before flooding with complete DMEM+FBS medium. The incubation was continued for 72 h. On the next day, the samples containing the HaCaT cells were washed with PBS and fixed on the surface of the sample by treating them with 2.5% glutaraldehyde for 2 h. The fixed cells were completely dehydrated with ethanol by gradually upgrading ethanol’s concentration. Samples were then treated with hexamethyldisilazane and were kept in desiccator for 30 min to dry it completely for SEM images.
Cell proliferation assay
Immortal human keratinocytes cells (HaCaT cells) were used for cell proliferation study on the modified polymeric substrates applying Alamar blue assay. Briefly, 20 μL (containing 104 cells) of Hacat cell suspension was applied on different polymeric substrates and control sheets (1 cm × 1 cm size) and incubated in humidified CO2 incubator (37°C, 5% CO2) for 3 h before flooding with complete DMEM+FBS medium. The incubation was continued for 7 days with media change on every alternate day. For Alamar blue assay, working solution was prepared by adding 10% Alamar blue solution in incomplete DMEM (without FBS) separately and this working solution was added to the cells by replacing the old media followed by incubation at 37°C in 5% CO2 incubator for 4 h period. After incubation culture supernatant was collected and OD was measured at 570 nm and 600 nm. The calculations were carried out according to the manufacturer's instructions.
Surface analysis
Surface functionality study
The AAm grafting on the LDPE substrate surface was monitored using ATR-FTIR spectroscopy which is illustrated in Figure 1a and 1b. Comparing the FTIR spectra of pristine LDPE and AAm-g-LDPE (Figure 1a), a broad absorption band at 3434 cm-1 was observed, which was due to the N-H stretching of NH2 group in AAm-g-LDPE. Strong bands appeared at around 1672-1633 cm-1 due to the C=O stretching (amide-II), respectively, indicate the introduction of amide group on the surface of LDPE. These characteristic peaks are absent in pristine LDPE film. As observed, from the spectra the C=O peak intensity in AAm-g-LDPE films was increased progressively with the increase of concentration and grafting time. This increase of C=O intensity indicates the increase of amide groups on the grafted LDPE.
Figure 1: ATR-FTIR characterizations: (a) Variation in CI values with different AAm concentrations at 3 h grafting time. Inset graph, ATR-FTIR spectra of pristine LDPE and AAm grafting onto LDPE (AAm-g-LDPE) with different AAm concentrations at 3 h grafting time, and (b) Variation in CI values with different grafting times at 0.25 w/v AAm concentration. Inset ATR-FTIR spectra of pristine LDPE and AAm-g-LDPE with different grafting times at 0.25 w/v AAm. Pristine LDPE film thickness = 100 μm.
In order to quantify the degree of AAm grafting on the LDPE surface the carbonyl index (CI) values were calculated from the FTIR spectra. The CI value was evaluated by taking the ratio of the integrated area of the peaks for C=O (~1672-1633 cm-1) and C-H bending of methylene unit (~729-719 cm-1).
The CI value was gradually increased up to 0.25 w/v AAm concentration as shown in Figure 1a and CI data are illustrated in Table 1. The increasing trend of CI value was not significant from 0.25 - 0.50 w/v AAm concentration. The above observation indicates the saturation of AAm on the grafted LDPE surface. The gradual increment of CI value was also observed with increase of grafting times. The experimental CI values were plotted against different grafting times for a fixed concentration of 0.25 w/v AAm in Figure 1b. Thus it may be said that with higher grafting time, more amount of AAm grafting occurred on LDPE surface. The values of grafting yield (%) are illustrated in Tables 1 and 2. For both cases, the grafting yield (%) of AAm followed a similar increasing trend as found with CI values.
Surface roughness analysis
Generally, chemical grafting can change the morphology of the polymer surfaces and produce the surface roughness. Morphology of the grafted surface was examined using SEM. To reveal the role of chemical grafting on the surface roughness of LDPE, SEM micrographs of grafted sample were compared with that of the pristine LDPE in Figures 2a and 2b. It is observed that the pristine LDPE film surface represents the smooth surface without any irregularity and roughness, while the AAm grafted films become rough due to the insertion of AAm onto LDPE backbone. For further topographic study, the grafted film surface (formation of microstructure) was investigated using AFM. In general, SEM gives general structural information (morphology) about the surface; AFM elucidates the detailed micro structure (topography).
AFM is a suitable tool to obtain 3D representation of surface topography [33,34]. In topographic analysis, the surface texture is measured with the extent of roughness. Roughness is quantified through vertical deviations (formation of the island like structure) of an actual surface from its real form. If the deviations are large, it represents the AAm moieties on the surface; in contrast no deviations show the surface smoothness. In Figure 2b (I), 3D presentation of pristine LDPE shows no deviation on the surface and its Rrms value was found be 19.7. 3D-AFM images in Figure 2b (II-VI) of AAm-g-LDPE exhibited significant change and the island like structure formation was observed with different grafting time. The Rrms values were found to be 27.3, 37.8, 42.2, 57.1 and 63.1 for 3, 5, 9, 13 and 19 h grafted LDPE specimen. The increase in island like structure was assumed to increase the number of attachment of grafted (AAm) chains on the main polymer backbone. These results revealed that the surface morphology is significantly changed via grafting. The grafting of hydrophilic AAm groups on the polyolefin surface may be helpful the bio interaction for cell adhesion and proliferation, and immobilization of active biomolecules (enzymes, thrombo-resistant agents, drug, etc.) [35].
Surface wettability analysis
In the preceding section, FTIR analysis revealed the grafting of AAm on the substrate surface. SEM and AFM analysis has shown the formation of roughness due to the grafting of AAm on the surface. Further, the water contact angle measurements were carried out to evaluate the wettability (hydrophilicity) of the functionalized surface. Figure 3a represents a comparison of contact angle of water droplets on pristine LDPE and AAm-g-LDPE surfaces. The contact angle of pristine LDPE (smooth surface) is 97°± 3° while for grafted surface (rough surface) the contact angle is dramatically decreased to 45°± 2°. These results suggest a positive impact of roughness of the grafted film surface because of the introduction of AAm moieties on the surface. From Figure 3b, it is clear that the wettability of the LDPE film is enhanced greatly by grafting AAm onto the surface. Before grafting, water contact angle of pristine LDPE film was found to be 97° ± 2° [36]. Surface contact angle values linearly dropped from 90° ± 3° to 45° ± 2°as the grafting time was allowed for 3,5,9,13 and 19 h at 0.25 w/v of AAm concentration. After 13 h grafting of AAm, the grafted specimen does not show any significant change in contact angle. It was assumed that the contact angle reached at equilibrium and finally the angle did not further change with time.
It is evident that the introduction of amine-based polar functionalities (AAm) on the LDPE surface, it tends to change from hydrophobic surface to hydrophilic. There might be the attachment of grafted chains on LDPE surface lead to development of hydrophilicity at the same time the surface morphology of the grafted LDPE film was altered as observed in SEM and AFM. Surface morphology also plays a vital role in water contact angle if the surface is rough and water penetrates into the roughness.
For better understanding of the correlation between water contact angle and surface roughness we have studied the Wenzel Model [37]. Wenzel equation described the wetting of a rough surface and hydrophilicity.
cosθ * = r cosθ
Here, θ is the contact angle of smooth surface, θ* is the contact angle of the rough surface, and r is the roughness factor, defined as the ratio of actual area to the apparent surface area of the substrate (Figure 4).
As r is always greater than 1, the roughness can amplify the hydrophilicity of a hydrophilic surface and the hydrophobicity of a hydrophobic surface [38]. As the 3D AFM image depicts the island like AAm graft chains with certain length, hence the water droplets interact with hydrophilic groups and penetrate into the cavities developed on the surface. When the pristine and grafted LDPE were exposed to water, the contact angle value of the functionalized surface was decreased from 97° to 45°, which indicate the hydrophilic nature of grafted LDPE. This result agrees with the prediction of the Wenzel equation, because the value of cos 90° is negative. According to the Wenzel model the water droplet penetrate into the cavity like structure.
Mechanical property analysis
To determine the effect of AAm grafting on the mechanical properties (such as tensile strength, % elongation at break) of the surface functionalized LDPE films, tensile testing of samples were done using UTM. Figure 5 represents the effect of grafting time on tensile properties of samples. For each sample an average of five tensile measurements were taken. The histograms show that there is no significant change in tensile strength and the elongation at break (%) of surface functionalized LDPE compared to pristine LDPE.
Nanomechanical property analysis
It has been analysed in above discussion of conventional testing where the bulk properties such as tensile strength, % elongation at break of the sample was not distinguished the influence of AAm grafting. Further, to investigation the nanomechanical properties such as the micro-hardness, reduced modulus and stiffness of the grafted LDPE surface was performed with continuous instrumented indentation tests (quasi-static nanoindentation) using Oliver-Pharr method [39]. Figure 6 represents the force-displacement curves for the pristine LDPE and AAm-g-LDPE with applied force of 400 μN. Generally, the loading causes both elastic and plastic deformation in the indentation the other hand unloading is led by recovery of the elastic deformation. From Figure 6 it can be seen ascend of loading curve increased in AAm-g-LDPE, which infers that the force required to produce same depth of indent in the test sample with AAm. As it is known the LDPE has amorphous and crystalline regions, therefore AAm easily diffuses into amorphous region due to low density (present a free volume) of LDPE hence loading curve rises in case of AAm-g-LDPE film. The resulting mechanical parameters and corresponding contact depth are summarized in Table 3. A mean value of ten measurements and standard deviation (SD) are used for the hardness, reduced modulus and stiffness. It can be seen the mechanical parameters show a slight change in case of AAm-g-LDPE film. It indicates that grafted surface is resist to penetration on the grafted surface, therefore, display a smaller displacement (greater recovery) than the pristine LDPE surface. Compare to pristine LDPE for AAm-g-LDPE the maximum contact depth obtained for a 400 μN load was 502 nm, indicating only a small influence of the AAm grafting on the nanomechanical parameters.
Cell adhesion and proliferation study
The surface properties (hydrophilicity, surface energy and surface roughness) play a significant role for the cell adhesion, proliferation and morphology of adhered cells. The cytocompatibility of the samples were tested to ensure that the steps followed for the functionalization did not render toxic products. Figures 7a and b show the cell adhesion and proliferation behaviour on the control, pristine LDPE and grafted LDPE surfaces with different duration of cell culture.
Figure 7: (a) Cell adhesion (HaCaT cells) in control, pristine LDPE and MA-g-LDPE film surface (number of cell seeded 5 ×104 cm-2) (b) Cell proliferation with time for control, pristine LDPE and MA-g-LDPE film surface (n=3). The results are shown as the mean ± SD performed in triplicates. (*P< 0.05 as compared to control).
In Figure 7a it can be seen that the cells were more adhered on the grafted surface than the pristine LDPE surface with culture time. Here the contact angle and surface topography also play a role for cell adhesion. As can be seen in Figure 3 that the contact angle decreased with the increase of grafting time, surface roughness increased with increasing grafting time (Figure 2). The water contact angle of AAm grafted LDPE was decreased to 45° from that of pristine LDPE (about 97°).
Therefore, in case of 19 h grafted sample, more number of cell adhesion occurred due to the presence of more number of amide groups on the surface. Amide groups show the better cell adhesion, probably due to hydrogen bonding between the surface amide group of the LDPE and polar groups of cell surfaces [40,41]. Figure 7b shows the cell proliferation (cell viability) on the pristine as well as grafted LDPE surface with different culture times (1,3,5,7 and 9 days). As observed, the cell viability on the surface increased and then decreased. On the first day of culture, cell proliferations were less for all the modified specimens. After 3 days of culture, the cell proliferation was highly increased up to 5 days on the grafted surface. This might happen due to the presence of more number of hydrophilic moieties on the surface. Consequently the cell proliferation was decreased for control and pristine LDPE film after 5 days cell culture time, which is similar to the general phenomena of cell culture on the substrate. Whereas the 19 h grafted sample (up to 7 and 9 days) still shows the high cell proliferation demonstrating the grafting stability of the samples. The cell proliferation on the grafted sample was found to be >75%. It is, therefore, reasonable to infer that no toxic contaminants leach out from the control as well as the synthesized specimens and that the samples are biocompatible. The SEM micrographs of the cell adherence and morphology of the pristine and grafted LDPE surface are shown in Figures 8a-8c. The cells retain their typical morphology and function during the entire culture period. The cells were found to grow over the samples to form a monolayer. The cell density on 9 h grafted samples was slightly greater than that on pristine LDPE, while on the 19 h grafted samples cells spreaded over the samples and formed a monolayer. These results revealed the biocompatibility of the samples.
In the present work, LDPE was chemically functionalized by AAm grafting on the surface using solution grafting method. We have shown that the surface modification of LDPE can be obtained in a controlled manner without significantly affecting the bulk properties of the film. It was found in contact angle measurement that the wettability of the modified samples was increased. It causes a decrease in contact angle value due to the introduction of polar (AAm) groups. The grafted surface was highly hydrophilic. Morphology analysis revealed the surface roughness was generated after the AAm grafting reaction on the LDPE surface. Nanomechanical property of the grafted film was slightly improved while the bulk properties remained same (as analyzed by tensile testing measurement). Alamar blue study of grafted samples with different time period showed the better cell adhesion and cell proliferation without affecting the cells shape and morphology. Development of hydrophilicity and surface roughness of AAm functionalized LDPE (AAm-g-LDPE) will be promising candidate for biomedical applications also the coating of other hydrophilic substance on the AAm-g-LDPE surface.
The authors are very thankful to Maulana Azad National Fellowship (UGC-CSIR) for financial support. The authors are also very grateful to Prof. T. K. Maiti for the help provided for Alamar blue assay test.