ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
S. Sreehari Sastry1,* and B. Rupa Venkateswara Rao2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
A series of 20CaO-10ZnO-59.9P2O5-10Pb3O4-0.1x, (x= V2O5, Cr2O3, MnO and CuO) glasses was prepared by melt quench technique. Spectroscopic properties of the prepared glass systems were investigated using XRay diffraction, Ultra-Violet (UV) spectroscopy, Differential Scanning Calorimetry (DSC), Electron Paramagnetic Resonance (EPR – X band), Fourier Transform Infra Red (FTIR) spectroscopy and Raman spectroscopy. Present investigation is useful to study the effect of glass composition on the optical, thermal, physical properties and symmetry of the prepared glasses. Various physical properties for all the glasses were measured. X-ray diffraction of glass samples was carried to check its amorphous nature. From EPR spectra, the spin-Hamiltonian parameters were calculated and these parameters were influenced by the change in glass composition for different transition metal ions. The FTIR spectral investigations of glasses revealed characteristic vibrations of PO4 3- units. The Raman spectra of all glass samples have displayed different spectral bands, and intensity of these bands. The results showed that the network structure of these glass samples is found to be change from glass to glass
Keywords |
| Phosphate Glasses, Differential scanning calorimetry, UV-Vis; EPR, FTIR, Raman spectral studies. |
INTRODUCTION |
| Modern technological applications have focused more attention in the studies of different types of glasses. Amongst wide variety glass systems, particularly, phosphate glasses are very important materials, because of their high thermal expansion coefficients, low optical dispersions and low glass transition temperature [1-3]. Phosphate glasses become particularly attractive in the field of optical technology in view of the fact that the fundamental optical absorption edge shift that would be caused by the introduction of metal oxide is the least in the case of phosphate glasses [4]. |
| The phosphate based glasses containing transition metal ions are of huge interest because of their superior properties, which arise from the presence of transition metal ions in multivalent states [5,6]. Phosphate glasses doped with transition metal ions find a place in the new lasers and luminescence materials [7]. Chemical durability of phosphate glasses greatly improved by addition of ZnO because Zn ion acts as an ionic cross linker between different phosphate anions, inhibiting hydration reaction. In the present study, the authors report the optical, thermal and site symmetry studies of transition metal ions in calcium lead zinc phosphate glasses. Incorporation of V2O5, Cr2O3, MnO and CuO is also expected to improve the chemical durability of the phosphate glasses. Therefore, it is interesting to study the effect of transition metal ions in calcium lead zinc phosphate glasses. |
EXPERIMENTAL |
| Materials |
| Glasses with compositions, 20CaO-10ZnO-59.9P2O5-10Pb3O4-0.1x, (x= V2O5, Cr2O3, MnO and CuO) were prepared using analytical grade compounds of CaO, P2O5, Pb3O4, ZnO, 0.1 mol% of V2O5, Cr2O3, MnO and CuO. These chemicals were thoroughly mixed and ground for 30 min in a mortar pastel and then the charge was melted in a porcelain crucible using furnace for 5 h at temperature ranging from 800-1100 0C depending on composition. When the melt was thoroughly homogenized and attained desirable viscosity, it was poured onto brass plate. The prepared glass was annealed at appropriate temperatures (between 300 and 400 0C) for 2 h and stored in desiccators prior to evaluation. The formulae and process applied here to resolve the physical properties in the study were put to test in earlier works of the authors [8]. |
| Experimental |
| XRD patterns for the glass powders were recorded in the 2θ range of 10-70° on a computer controlled X-ray diffractometer (Model PW1170) with Cu Kα (λ=1.5418 A°) source. The differential scanning calorimetry (DSC) measurements were performed using Netzsch DSC 204 instrument. About 5-10 mg of the material was taken in an aluminum pan of the DSC setup and scanned at a heating rate of 10 oC/min. The UV and visible optical transmissions for glass samples were measured by using JASCO model V-670 UV-Vis-NIR spectrophotometer covering the range 200-900 nm. EPR spectra were recorded at room temperature through BRUKER-ER073 series in the X-band frequency (9.4 GHz) at 100 kHz field modulation. The magnetic field was scanned from 0 to 800 mT and the microwave power used was 1mW. The IR infrared absorption spectra of the glasses were recorded at room temperature in the wave number range 4000–400 cm-1 by SHIMADZU 8201 PC FT-IR spectrophotometer. The glass samples were subjected to Raman spectra in the range 200-3500 cm-1 with Lab RAM HR-800-HORIBAJOBINYVON Raman spectrometer. |
RESULTS AND DISCUSSION |
| Figure 1 shows XRD patterns of calcium lead zinc phosphate glass system compositions. This figure shows a broad diffuse scattering at different angles instead of crystalline peaks (absence of Bragg Peaks) and no continuous or discrete sharp peak, which reflect the characteristics of amorphous glass structure. |
 |
| Differential scanning calorimetry (DSC) is one of the most frequently used thermal analysis technique. DSC monitors heat effects allied with the phase transitions and chemical reactions as a role of temperature. DSC thermograms of present glass system are shown in Figure 2. |
 |
 |
| From Table 2, it can be seen that change in the average molecular weight M, density significantly influences the physical properties such as dielectric constant, reflection losses, polaron radius, inter-ionic distance and electronic polarizability. From the data presented in Table 2, it can also be seen that, the physical properties are changing from glass to glass and hence the environment around the transition metal ions in these glasses also changes from glass to glass. The ideal values of optical basicity can be predicted from composition of glass and basicity moderating parameters of various cations present [9]. Basicity parameter slightly increases from glass TM1 to TM4. High optical basicity means high electron donor ability of oxide ions to the cations. Understanding of optical basicity is useful for the design of the novel optical functional materials like polarizerâÃâ¬ÃŸs, detectors and modulators with higher optical performances [10]. However, during the formation of glasses the orientation, arrangement and distribution of transition metal ions may differ in different glasses, which lead to different structural arrangements and bonding. |
| The absorption spectra of vanadium containing glasses are of very complex nature because of presence of three valance states of vanadium. In the present study of glasses, V2O5 doped glass attained the faint green colour and exhibited absorption bands at about 685 and 829 nm, as shown in Fig. 3(a). These absorption bands correspond to 2B2g → 2B1g , 2B2g → 2Eg transitions, a characteristic of VO2+ ions [11]. The assignment of these bands has been made on the basis of an energy level scheme for molecular orbitals of VO2+ ion in a ligand field of C4v symmetry [12]. VO2+ has never exhibited an ideal octahedral symmetry, but lowers to tetragonal (C4v) [13]. Cr2O3 doped glass attained light green colour and the absorption bands appeared in the visible region nearly at 460, 630,660 and 690 nm, as shown in Fig. 3(b). Using Tanabe-Sugano diagrams for d3 ions, the observed optical absorption bands are assigned to 4A2g(F) → 2T1g (F), 4A2g(F) → 2T1g(G), 4A2g(F) → 4T2g(F) and 4A2g(F) → 2Eg(G). The observed bands indicated that Cr3+ ions are present mostly in distorted octahedral coordination [14]. The presence of octahedral site is assumed to be related to the strong field stabilization energy of Cr3+ ions in sixfold coordination [15]. The UV- Visible absorption spectra of MnO doped lead zinc phosphate glass is shown in Fig. 3(c). The observed bands nearly at around 370, 406, 448 and 532 are assigned to the transitions 6A1g(S) → 4T1g (G), 6A1g(S) →4T2g(G), 6A1g(S) → 4A1g (G)+4Eg (G) and 6A1g(S) →4T2g (D), characteristic of Mn2+ ions in octahedral symmetry [16,17]. This analysis shows that manganese ions exist mainly in Mn2+ state, and occupy tetrahedral positions. The optical absorption spectrum is influenced by the host structure into which the transition metal ions are incorporated. In oxide glasses, the transition metal ions, mostly, form coordination complexes with doubly charged oxygen as the ligands. The optical absorption spectra for CuO doped glass is shown in Fig. 3(d). The optical absorption spectrum of the glass containing Cu2+ ions shows in a broad absorption band at about 825 nm, which can be attributed to the presence of Cu2+ ion and the octahedron being tetragonally distorted [18]. This absorption can be assigned to the 2Eg(D) → 2T2g(D) transition of Cu2+ ion. |
 |
| The cubic symmetry of Cu2+ ions is disturbed by electronic hole in the degenerate orbital which has caused the tetragonal distortion. However, Cu2+, being as d9 ion, experiences a strong Jahn-Teller distortion, which leads to the splitting of energy levels [18,20] and causes predominantly an elongated octahedral coordination with four short inplane bond lengths and longer axial bond lengths. Table 3 shows the observed optical absorption band positions and the transitions of prepared glass samples. When all these absorption bands are correlated, the maximum absorption band observed is in case of Cu2+ doped glass while the remaining glasses contain more number of absorption bands. |
 |
 |
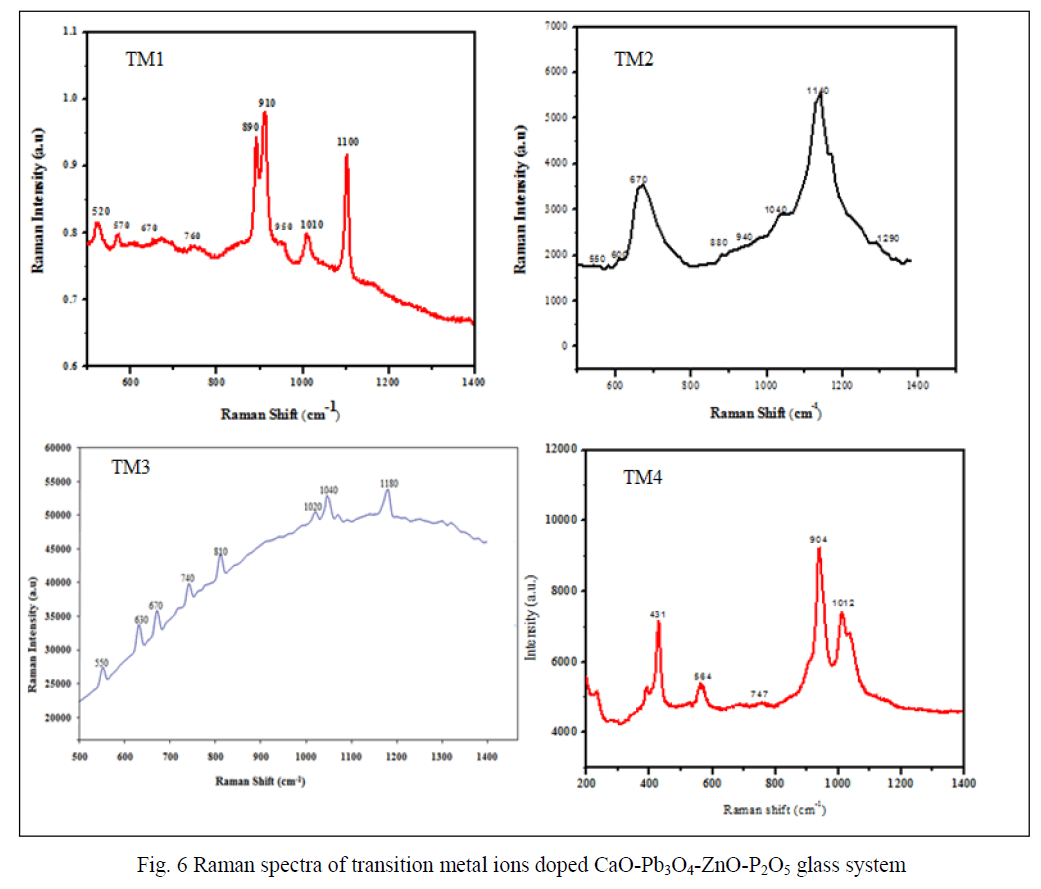
| Figure 5 shows the infrared spectra of the present glass samples. The FTIR spectra exhibited several peaks, which can be divided as medium and broad. Similar to other phosphate glasses, the present samples also exhibited the main characteristic active vibrational modes of phosphate network in the range 1400 – 600 cm-1. The bands at 1180 cm-1 are assigned to the vibrations of PO3 2- groups at the end of chains [25]. The bands observed at < 600 cm-1 are related to phosphate network bending vibrations [26]. The bands at ~1080 cm-1 are due to PO4 3- fundamental vibrational modes. Band at 1020 cm-1 is due to the symmetric stretching vibrations of the (PO4 3-) tetrahedra (P–O- ionic group) [27]. The fundamental band at 980 cm-1 is observed in case of all samples and it is an indication of phosphate ions in p state, exist in tetrahedral symmetry and the bands in 950 cm-1 region are due to the asymmetric stretching vibrations of P–O– P groups [28]. Bands at 720 and 730 cm-1 are due to symmetric vibration the P-O-P chains. FTIR spectroscopy establishes the type of structural units, whose number increases with the successive replacement of the TM content. |
 |
 |
CONCLUSIONS |
| The structures of studied glasses can be considered as phosphate units connected with VO2+, Cr3+, Mn2+ and Cu2+ ions in tetragonally distorted octahedral sites. DSC results indicate that vanadium ions increase cross-link density and enhance mean bond strength in TM1 glass. From the optical absorption spectra, the broad and a sharp absorption band observed in Cu2+ doped glass while the remaining glasses contain more number of absorption bands. EPR results conclude that the spin-Hamiltonian parameters are influenced by the change in the glass composition with different transition metal ions. FTIR and Raman spectra exhibited the main characteristic active vibrational modes of phosphate network and successive replacement of transition metal oxides leads to change in site symmetry of the prepared glasses. |
ACKNOWLEDGMENTS |
| The authors gratefully acknowledge University Grants Commission Departmental Research Scheme at Level III program No. F.530/1/DRS/2009 (SAP-1), dated 9 February 2009, and Department of Science and Technology -Fund for Improving Science and Technology program No. DST/FIST/PSI–002/2011 dated 20-12-2011, New Delhi, to the department of Physics, Acharya Nagarjuna University for providing financial assistance. |
References |
|