Keywords
|
| Norfloxacin, Europium, Conductivity, Infrared, Thermal |
INTRODUCTION
|
| Metalloantibiotics can interact with several different kinds of biomolecules, including DNA, RNA, proteins, receptors, and lipids, rendering their unique and specific bioactivities. Antibiotics that interact with metal ions constituted a class of drugs which has been widely used in medicine both for human beings and animals [1, 2]. Many drugs possess modified pharmacological and toxicological properties when administered in the form of metallic complexes. Probably the most widely studied cation in this respect is Cu(II), for which a host of low-molecular-weight Cu(II) complexes have been proved beneficial against several diseases such as tuberculosis, rheumatoid arthritis, gastric ulcers, and cancers [3, 4]. Norfloxacin is considered the best of the third generation quinolone family. There are several reports regarding the synthesis and crystal structure of metal complexes with quinolone derivatives [5, 6]. Quinolone antibiotics could participate in the formation of complexes in a number of ways [7, 8]. When in acidic media, quinolones are usually singly and/or doubly protonated making them unable to coordinate to the metal cations and, in such cases, only electrostatic interaction are observed between the drug and the metal ions [9]. There are several metal– NFX complexes and their biological activities studies have been reported [23-28]. This paper was discussed the interaction behavior between the europium(III) and NFX antibiotic ligand using infrared and thermal tools. |
II. EXPERIMENTAL
|
| The EuCl3.6H2O, NFX (Fig. 1) and methanol solvent were obtained from Aldrich Company. All chemicals used in this study were of analytically reagent grade and used without further purification. The 3 mmol of NFX suspended in 50 mL of methanol was mixed with another solution containing 1 mmol of the EuCl3.6H2O in 25 mL distilled water. The reaction mixture was then basified by adding ammonia solution (1 M) and was refluxed at ~ 80 °C for about 6 h. The product obtained as a precipitate was collected by filtration and washed with a mixture of methanol/water (50/50; v/v). The product thus obtained was dried (80 °C) under vacuum over anhydrous calcium chloride. Elemental analyses of carbon, hydrogen and nitrogen elements were analyzed by a Perkin-Elmer CHN 2400 elemental analyzer. The europium metal content was calculated gravimetrically by converting the europium-NFX complex to its corresponding Eu2O3 oxide. The molar conductivity of freshly prepared 1.0×10-3 mol/cm3 dimethylsulfoxide solutions were measured for the soluble complex using Jenway 4010 conductivity meter. The infrared spectra, as KBr discs, were recorded on a Bruker FT-IR Spectrophotometer (4000–400 cm-1). The thermal study was carried out on a Shimadzu thermogravimetric analyzer at a heating rate of 10 oC min-1 under nitrogen till 800 oC. |
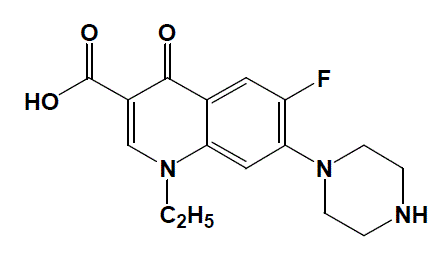 |
| Fig. 1: The structural formula of NFX antibiotic drug |
III. RESULTS AND DISUSSION
|
| The europium-NFX complex with formula [Eu(NFX)3].6H2O was assigned under the interaction between Eu(III) chlorides and NFX drug with 1:3 molar ratio. This resulted complex was stable solid at room temperature. Testing for chloride ions held as follows; the solid complex was acidified by adding few drops of concentrated nitric acid. The nitric acid reacts with, and removes, other ions that might also give a confusing precipitate with silver nitrate. The basis of the test is that a solution containing chloride ions changes immediately into a whitish solution when silver nitrate solution is added (0.1 mol/L). If these chloride ions are not present, the solution remains clear and transparent. The molar conductance of the isolated complex indicated that this complex was non-electrolytes [10] which agreed with the elemental analysis data and the absence of chloride ions. Elemental analysis data of the white Eu(III)–NFX complex: Calc. %C:47.45; %H: 5.23; %N: 10.38; %Eu: 12.51, Found. %C: 45.95; %H: 5.21; %N: 9.86; %Eu: 12.37. |
| The infrared spectrum of the prepared Eu(III)-NFX complex has been represented in Fig. 2. The NFX ligand showed strong broad band near at 3400 cm-1. This band could be assigned to the stretching vibration of the ν(N–H) group. This band existed in the spectrum of the Eu(III) complex that indicate the non-participation of this group in the coordination process with metal ions [11]. The free NFX ligand has a two strong bands located at 1620 and 1727 cm-1 which assigned to the stretching vibration of the carboxylic ν(COOH) and the carbonyl ν(CO) groups [12]. Insight into the spectrum of NFX, it was found that the absence of the νCOOH stretching vibration, explains strongly the deprotonation of NFX ligand [11]. In the literature survey [12] a study which deals with the location of both asymmetric (1585 cm–1) and symmetric (1380 cm–1) stretching depending upon the deprotonation of carboxylate group. The main infrared spectral bands detectable for the NFX ligand in the Eu(III)-NFX complex confirm the deprotonation situation for the Eu(III)-NFX complex. In the chelation process, the carbonyl ν(CO) group was disappeared and the ν(COOH) stretching vibration in NFX ligand appeared at (1617 and 1616) cm–1 and (1335 and 1383) cm–1 due to the asymmetric and symmetric stretching vibration of the ligated COO– group, respectively [11, 12]. The [Δν = (νasym COO–– νsym COO–) in case of Eu(III) complex was observed at 238 cm–1, which suggested a uni–dentate interaction of the carboxylate group toward the central metal ions. The other reason, which confirms the place of coordination is that the disappeared of carbonyl group ν(C=O) which presence at 1727 cm–1 for the free NFX ligand. The new bands were existed in Eu(III)-NFX complex at 553, 523, 506 and 474 cm–1 assigned to M-O stretching vibration motion [45, 46] of Eu(III) O-carboxylate and Eu(III) O-carbonyl groups. The uncoordinated water molecules were observed at the ~ 3400 cm–1. The suggested chelation of NFX ligand toward Eu(III) metal ion can be illustrated in Fig. 3. |
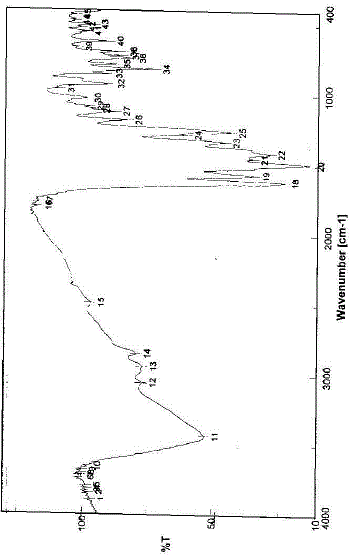 |
| Fig. 2: Infrared spectrum of Eu(III)–NFX complex |
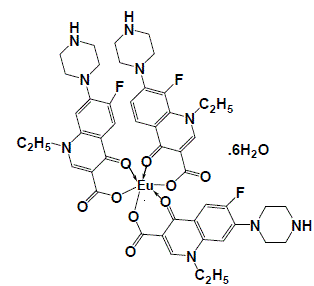 |
| Fig. 3: Suggestion structure of [Eu(NFX)3].6H2O complex |
| Figure 4 show the TG/DTG curves of [Eu(NFXr)3].6H2O under nitrogen atmosphere. From this Figure, the following results can be discussed. The TG/DTG curves of the Eu(III)-NFX hydrated complexes show four cconsecutives and successive decomposition steps (DTGmax = 141, 251, 336 and 603 oC) with total weight loss 85.89%, it’s so difficult to be separated. Logically the first step responsible to the dehydration process and the others are assigned to the decomposition process for the NFX chelating ligands. The processes of dehydration and decomposition of NFX ligand are accompanied by endothermic peaks. The final residue of decomposition of the [Eu(NFXr)3].6H2O complex is europium(III) oxide with percentage 14.11%. The result of ½Eu2O3 residue was agreed with that of calculated percentage 14.48%. The three terminal of ethyl groups and fluorine atoms of the three NFX moieties are the early groups which were loosed in the form of ethylene and hydrogen fluoride molecules, respectively. The Eu2O3 oxide was stable in the temperature range 710–800 oC. |
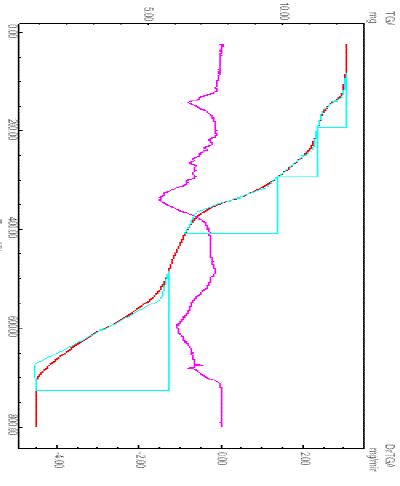 |
| Fig. 4: TG/DTG curves of Eu(III)–NFX complex |
References
|
- Alipio BC, David FC, Isabel RS, Juan CNM. Manuel AE. Comparison of batch, stirred flow chamber, and column experiments to study adsorption, Desorption and transport of carbofuran within two acidic soils. Chemosphere. 2012; 88(1): 106-120.
- Arnaud B, Richard C, Michel S. A comparison of five pesticides adsorption and Desorption processes in thirteen contrasting field soils. Chemosphere. 2005; 61(5): 668-676.
- Chunxian W, Jin-Jun W, Su-Zhi Z, Zhong-Ming Z. Adsorption and Desorption of Methiopyrsulfuron in Soils. Pedosphere. 2011; 21(3): 380-388.
- Chunxian W, Suzhi Z, Guo N, Zhongming Z, Jinjun W. Adsorption and Desorption of herbicide monosulfuron-ester in Chinese soils. J Environ Sci. 2011; 23(9): 1524-1532.
- Christine MFB, Josette MF. Adsorption-desorption and leaching of phenylurea herbicides on soils. Talanta. 1996; 43(10): 1793-1802.
|